| In vitro: |
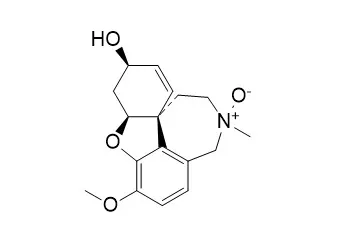
| Molecules . 2011 Nov 15;16(11):9520-33 | | Acetylcholinesterase-inhibiting alkaloids from Zephyranthes concolor[Pubmed: 22086403] | | Chlidanthine and galanthamine N-oxide inhibited electric eel acetylcholinesterase (2.4 and 2.6 × 10(-5) M, respectively), indicating they are about five times less potent than galanthamine, while galwesine was inactive at 10(-3) M. Inhibitory activity of HIV-1 replication, and cytotoxicity of the isolated alkaloids were evaluated in human MT-4 cells; however, the alkaloids showed poor activity as compared with standard anti-HIV drugs, but most of them were not cytotoxic. | | Drug Metab Dispos . 2002 May;30(5):553-63. | | The metabolism and excretion of galantamine in rats, dogs, and humans[Pubmed: 11950787] | | Galantamine is a competitive acetylcholine esterase inhibitor with a beneficial therapeutic effect in patients with Alzheimer's disease. The metabolism and excretion of orally administered (3)H-labeled galantamine was investigated in rats and dogs at a dose of 2.5 mg base-Eq/kg body weight and in humans at a dose of 4 mg base-Eq. Both poor and extensive metabolizers of CYP2D6 were included in the human study. Urine, feces, and plasma samples were collected for up to 96 h (rats) or 168 h (dogs and humans) after dosing. The radioactivity of the samples and the concentrations of galantamine and its major metabolites were analyzed. In all species, galantamine and its metabolites were predominantly excreted in the urine (from 60% in male rats to 93% in humans). Excretion of radioactivity was rapid and nearly complete at 96 h after dosing in all species. Major metabolic pathways were glucuronidation, O-demethylation, N-demethylation, N-oxidation, and epimerization. All metabolic pathways observed in humans occurred in at least one animal species. In extensive metabolizers for CYP2D6, urinary metabolites resulting from O-demethylation represented 33.2% of the dose compared with 5.2% in poor metabolizers, which showed correspondingly higher urinary excretion of unchanged galantamine and its N-oxide. The glucuronide of O-desmethyl-galantamine represented up to 19% of the plasma radioactivity in extensive metabolizers but could not be detected in poor metabolizers. Nonvolatile radioactivity and unchanged galantamine plasma kinetics were similar for poor and extensive metabolizers. Genetic polymorphism in the expression of CYP2D6 is not expected to affect the pharmacodynamics of galantamine. | | Phytochem Anal . 2018 Mar;29(2):217-227. | | Alkaloids of Amaryllidaceae as Inhibitors of Cholinesterases (AChEs and BChEs): An Integrated Bioguided Study[Pubmed: 29044771] | | Introduction: Enzymatic inhibition of acetylcholinesterase (AChE) is an essential therapeutic target for the treatment of Alzheimer's disease (AD) and AChE inhibitors are the first-line drugs for it treatment. However, butyrylcholinesterase (BChE), contributes critically to cholinergic dysfunction associated with AD. Thus, the development of novel therapeutics may involve the inhibition of both cholinesterase enzymes.
Objective: To evaluate, in an integrated bioguided study, cholinesterases alkaloidal inhibitors of Amaryllidaceae species.
Methodology: The proposed method combines high-performance thin-layer chromatography (HPTLC) with data analysis by densitometry, enzymatic bioautography with different AChEs and BChEs, the detection of bioactive molecules through gas chromatography mass spectrometry (GC-MS) analysis of spots of interest, and theoretical in silico studies.
Results: To evaluate the bioguided method, the AChE and BChE inhibitory activities of seven Amaryllidaceae plant extracts were evaluated. The alkaloid extracts of Eucharis bonplandii exhibited a high level of inhibitory activity (IC50 = 0.72 ± 0.05 μg/mL) against human recombinant AChE (hAChE). Regarding human serum BChE (hBChE), the bulb and leaf extracts of Crinum jagus had the highest activity (IC50 = 8.51 ± 0.56 μg/mL and 11.04 ± 1.21 μg/mL, respectively). In the HPTLC spots with high inhibitory activity, several alkaloids were detected using GC-MS, and some of these alkaloids were identified. Galanthamine, galanthamine N-oxide and powelline should be the most prominent inhibitors of substrate accommodation in the active site of the Torpedo californica AChE (TcAChE), hAChE and hBChE enzymes.
Conclusions: These results are evidence of the chemical relevance of the Colombian's Amaryllidaceae species for the inhibition of cholinesterases and as potent sources for the palliative treatment of AD. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)