| Description: |
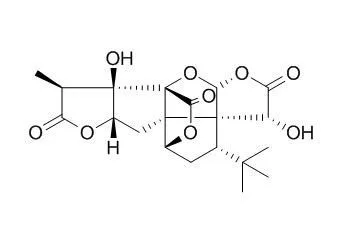
Ginkgolide A is a platelet-activating factor antagonist, it can inhibit the neurotoxicity of prions or amyloid-beta1-42, may be relevant treatments for prion or Alzheimer's diseases.Ginkgolide A has neuroprotective, and anxiolytic-like effects, it is widely used for the treatment of cardiovascular diseases and diabetic vascular complications, which might be achieved through regulating the STAT3-mediated pathway. |
| Targets: |
STAT | IL Receptor | PI3K | Akt | GSK-3 | P450 (e.g. CYP17) | Caspase | Beta Amyloid |
| In vitro: |
| Int Immunopharmacol. 2015 Apr;25(2):242-8. | | Ginkgolide A reduces inflammatory response in high-glucose-stimulated human umbilical vein endothelial cells through STAT3-mediated pathway.[Pubmed: 25681539] | High-glucose-induced low-grade inflammation has been regarded as a key event in the onset and progression of endothelial dysfunction in diabetic vascular complications. Ginkgolide A (GA), a major compound from Ginkgo biloba extract, is widely used for the treatment of cardiovascular diseases and diabetic vascular complications.
Here, its effect on high-glucose-stimulated vascular inflammation in human umbilical vein endothelial cells (HUVECs) was investigated.
METHODS AND RESULTS:
In the present study, the optimal stimulation conditions for HUVECs were screened for inducing endothelial inflammation, namely, high glucose at the concentration of 30mM for continuous 8h. The endothelial production of high-glucose-induced interleukin (IL)-4, IL-6, IL-13 and signal transducer and activator of transcription-3 (STAT-3) phosphorylation were significantly inhibited by the pretreatment with GA at concentrations of 10, 15 and 20μM based on enzyme-linked immunosorbent assay (ELISA), western blot or/and RT-PCR experiments. These senescent alterations induced by high glucose were significantly attenuated by the specific STAT3 inhibitor S3I-201 at the concentration of 20μM. Furthermore, the phosphorylation of STAT3, IL-4, IL-6, IL-13 and intercellular cell adhesion molecule-1 (ICAM-1) protein as well as mRNA levels were attenuated by the pretreatment of cells with STAT3 siRNA. Our results demonstrated that GA improved high-glucose-caused low-grade vascular inflammation, which might be achieved through regulating the STAT3-mediated pathway.
CONCLUSIONS:
These findings indicated that GA might be a promising candidate for attenuating vascular inflammation in diabetic vascular complications. |
|
| In vivo: |
| J Nat Prod. 2003 Oct;66(10):1333-7. | | An anxiolytic-like effect of Ginkgo biloba extract and its constituent, ginkgolide-A, in mice.[Pubmed: 14575433 ] | The anxiolytic-like effects of Ginkgo biloba extract (GBE) and its four terpenoid components (Ginkgolide A, ginkgolide B, ginkgolide C, and bilobalide) were assessed using the elevated plus-maze test in mice.
METHODS AND RESULTS:
Administration of GBE as a single oral dose (0.5 or 1 g/kg, po) caused a state of suppressed motor activity and, thus, shortened the time spent in the open-sided arms. However, when GBE (0.063-1 g/kg, po) was administered daily for 7 days and the plus-maze test was carried out 24 h after the final administration, the time spent in the open-sided arms was prolonged, with the peak anxiolytic-like effect at 0.125 g/kg. A combination of seven-day administration of GBE (0.125 g/kg) and a single dose of diazepam (1 mg/ kg, po, 10 min before testing) enhanced the anxiolytic-like effect. Flumazenil (0.3 mg/kg, ip, 10 min before testing) blocked the effect of diazepam, but not of GBE. Daily administration of Ginkgolide A (1 or 2 mg/kg, po) resulted in an anxiolytic-like effect by the third treatment, with the maximal effect observed after the fifth administration. Neither ginkgolide B, ginkgolide C, nor bilobalide produced any anxiolytic-like effects. At doses higher than 0.5 g/kg, GBE not only inhibited motor activity but also suppressed active avoidance behavior, reduced caffeine-induced stimulation, and enhanced pentobarbital-induced sleep, while Ginkgolide A (up to 20 mg/kg) did not exhibit these effects. Diazepam (1 mg/kg) is known to enhance pentobarbital-induced sleep.
CONCLUSIONS:
These results suggest that GBE produces a significant anxiolytic-like effect following repeated administration and that Ginkgolide A is most likely responsible for this effect. There are also indications that although GBE exerts a sedative effect at comparatively higher doses, Ginkgolide A has a relatively weak tendency to produce benzodiazepine-like side effects. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)