| Description: |
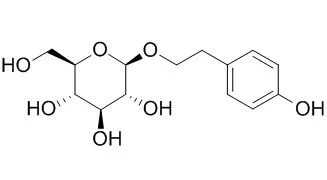
Salidroside is a prolyl endopeptidase inhibitor, which has cardioprotective, antidiabetic,antidepressant, anxiolytic, anti-tumor, and antioxidant actions. Salidroside alleviates cachexia symptoms in mouse models of cancer cachexia via activating mTOR signalling. It alleviates the pulmonary symptoms of paraquat-induced acute lung injury, at least partially, by repressing inflammatory cell infiltration and the expression of TGF-β1 resulting in delayed lung fibrosis; and it has protective effect against hypoxia-induced cardiomyocytes necrosis and apoptosis by increasing HIF-1α expression and subsequently up-regulating VEGF levels. It may be a potential therapeutic agent for treating or preventing neurodegenerative diseases implicated with oxidative stress. |
| Targets: |
VEGFR | JAK | STAT | MMP(e.g.TIMP) | PI3K | Akt | AMPK | GSK-3 | TNF-α | NF-kB | AP-1 | TGF-β/Smad | COX | NOS | Beta Amyloid |
| In vitro: |
| Int J Clin Exp Pathol. 2015 Jan 1;8(1):615-21. eCollection 2015. | | Anticancer effect of salidroside on colon cancer through inhibiting JAK2/STAT3 signaling pathway.[Pubmed: 25755753] | Salidroside is considered to have anti-tumor properties. We investigate its effects on colon carcinoma SW1116 cells.
METHODS AND RESULTS:
Cell viability was assessed by CCK-8. Propidium iodide (PI) staining was used to determine the cell cycle by flow cytometry. The migration and invasion were detected by Transwell. Western blot was used to detect the expression of STAT3 signal related proteins. As the result, high concentrations of Salidroside (10, 20. 50 μg/ml) significantly inhibited proliferation of SW1116 cells in a parallelly, cell cycle arrest was increased at the G0/G1 phase after Salidroside treatment. Furthermore, Salidroside inhibited migration and invasion of SW1116 cells. Salidroside treatment decreased proteins expression of phosphorylation levels in JAK2/STAT3 signaling, while MMP-2 and MMP-9 proteins levels were decreased and protein expression of VEGF and VEGFR-2 were down-regulated.
CONCLUSIONS:
In Conclusion, Salidroside inhibited proliferation, decreased the migration and invasion of SW1116 cells in JAK2/STAT3-dependent pathway, the specific mechanisms need further study. | | Front Pharmacol . 2017 Oct 18;8:749. | | Salidroside, A Natural Antioxidant, Improves β-Cell Survival and Function via Activating AMPK Pathway[Pubmed: 29093682] | | Abstract
Aim: The enhanced oxidative stress contributes to progression of type 2 diabetes mellitus (T2DM) and induces β-cell failure. Salidroside is a natural antioxidant extracted from medicinal food plant Rhodiola rosea. This study was aimed to evaluate protective effects of Salidroside on β-cells against diabetes associated oxidative stress. Methods and Results: In diabetic db/db and high-fat diet-induced mice, we found Salidroside ameliorated hyperglycemia and relieved oxidative stress. More importantly, Salidroside increased β-cell mass and β-cell replication of diabetic mice. Mechanism study in Min6 cells revealed that, under diabetic stimuli, Salidroside suppressed reactive oxygen species production and restore mitochondrial membrane potential (ΔΨm) via reducing NOX2 expression and inhibiting JNK-caspase 3 apoptotic cascade subsequently to protect β-cell survival. Simultaneously, diabetes associated oxidative stress also activated FOXO1 and triggered nuclear exclusion of PDX1 which resulted in β-cell dysfunction. This deleterious result was reversed by Salidroside by activating AMPK-AKT to inhibit FOXO1 and recover PDX1 nuclear localization. The efficacy of Salidroside in improving β-cell survival and function was further confirmed in isolated cultured mouse islets. Moreover, the protective effects of Salidroside on β-cells against diabetic stimuli can be abolished by an AMPK inhibitor compound C, which indicated functions of Salidroside on β-cells were AMPK activation dependent. Conclusion: These results confirmed beneficial metabolic effects of Salidroside and identified a novel role for Salidroside in preventing β-cell failure via AMPK activation. Our finding highlights the potential value of Rhodiola rosea as a dietary supplement for diabetes control.
Keywords: AMPK; oxidative stress; Salidroside; type 2 diabetes; β-cells. | | J Cachexia Sarcopenia Muscle . 2016 May;7(2):225-32. | | Salidroside alleviates cachexia symptoms in mouse models of cancer cachexia via activating mTOR signalling[Pubmed: 27493875] | | Abstract
Background: Cachexia has a devastating impact on survival and quality of life for many cancer patients and contributes to nearly one-third of all cancer deaths; also, it is associated with poor responses to chemotherapy and survival. A better understanding of the underlying mechanisms of cancer-associated cachexia (CAC), coupled with effective therapeutic approaches, will improve management of progressive functional impairment in cancer patients. Salidroside, a phenylpropanoid glycoside in Rhodiola rosea L, has been reported to possess potential anti-fatigue, anti-ageing, and anti-Alzheimer's disease properties. It is widely consumed as a nutritional supplement, but its effects on CAC and the possible mechanism remain a mystery.
Methods: In the murine models of cachexia induced by CT-26 and Lewis lung carcinoma (LLC) tumour, respectively, main features of CAC were determined after treatment of Salidroside or chemotherapy. In vitro experiments were performed using murine C2C12 myotubes, which were treated by tumour necrosis factor-α. Levels of several critical muscle-related signal proteins such as mammalian target of rapamycin (mTOR), p-mTOR, and myosin heavy chain (MyHC) were examined using western blot both in vitro and in vivo.
Results: In the present study, we showed the exciting effect of Salidroside on the treatment of CAC. In CT-26 and LLC models, respectively, Salidroside treatment could effectively preserve the tumour-free body weight, decrease loss of adipose and gastrocnemius muscles, alleviate tumour burden, and prolong their survival time. Additionally, in combined chemotherapy, Salidroside could synergistically enhance the anti-tumour activity of cisplatin, especially decreased or eliminated chemotherapy-induced cachexia. Further analysis demonstrated that Salidroside could significantly increase expression of mTOR, p-mTOR, and MyHC in gastrocnemius muscle. Also, results in vitro showed that Salidroside could not only obviously increase mTOR, p-mTOR, and MyHC expression in C2C12 myotubes but also effectively rescue their down-regulation induced by tumour necrosis factor-α.
Conclusions: In the current study, the exciting effect of Salidroside on CAC suggested that Salidroside supplementation might be a promising approach for a multi-targeted therapy for the treatment of CAC.
Keywords: Cancer‐associated cachexia; MyHC; Salidroside; Skeletal muscle; mTOR. | | Oxid Med Cell Longev . 2019 Sep 10;2019:9341018. | | Salidroside Protects Dopaminergic Neurons by Enhancing PINK1/Parkin-Mediated Mitophagy[Pubmed: 31583052] | | Abstract
Parkinson's disease (PD) is a common neurodegenerative disease characterized by the degeneration of nigrostriatal dopaminergic (DA) neurons. Our previous studies have suggested that Salidroside (Sal) might play neuroprotective effects against PD by preserving mitochondrial Complex I activity. However, the exact mechanism of the neuroprotective effect of Sal remains unclear. Growing evidence indicates that PINK1/Parkin-mediated mitophagy is involved in the development of PD. In this study, we investigated whether Sal exerts a neuroprotective effect by modulating PINK1/Parkin-mediated mitophagy. Results showed that Sal alleviated MPTP-induced motor deficits in pole test. Moreover, Sal diminished MPTP-induced degeneration of nigrostriatal DA neurons as evidenced by upregulated TH-positive neurons in the substantia nigra, increased DAT expression, and high dopamine and metabolite levels in the striatum. Furthermore, in comparison with the MPP+/MPTP group, Sal considerably increased the mitophagosome and mitophagy flux. Moreover, in comparison with the MPP+/MPTP group, Sal evidently enhanced the mitochondrial expression of PINK1 and Parkin, accompanied by an increase in the colocalization of mitochondria with Parkin. However, transfection of MN9D cells with PINK1 siRNA reversed Sal-induced activated mitophagy and cytoprotective effect. In conclusion, Sal may confer neuroprotective effects by enhancing PINK1/Parkin-mediated mitophagy in MPP+/MPTP-induced PD models. |
|
| In vivo: |
| Br J Pharmacol. 2015 Mar 5. | | Salidroside ameliorates insulin resistance through activation of a mitochondria-associated AMPK/PI3K/Akt/GSK3β pathway.[Pubmed: 25754463] | Recent reports have suggested that Salidroside could protect cardiomyocytes from oxidative injury and stimulate glucose uptake in skeletal muscle cells by activating AMP-activated protein kinase (AMPK). The aim of this study was to evaluate the therapeutic effects of Salidroside on diabetic mice and to explore the underlying mechanisms.
METHODS AND RESULTS:
The therapeutic effects of Salidroside on type 2 diabetes were investigated. Increasing doses of Salidroside (25, 50 and 100 mg·kg(-1) ·day(-1)) were administered p.o. to db/db mice for 8 weeks. Biochemical analysis and histopathological examinations were conducted to evaluate the therapeutic effects of Salidroside. Primary cultured mouse hepatocytes were used to further explore the underlying mechanisms in vitro.
Salidroside dramatically reduced blood glucose and serum insulin levels and alleviated insulin resistance. Hypolipidaemic effects and amelioration of liver steatosis were observed after Salidroside administration. In vitro, Salidroside dose-dependently induced an increase in the phosphorylations of AMPK and PI3K/Akt, as well as glycogen synthase kinase 3β (GSK3β) in hepatocytes. Furthermore, Salidroside-stimulated AMPK activation was found to suppress the expression of PEPCK and glucose-6-phosphatase. Salidroside-induced AMPK activation also resulted in phosphorylation of acetyl CoA carboxylase, which can reduce lipid accumulation in peripheral tissues. In isolated mitochondria, Salidroside inhibited respiratory chain complex I and disturbed oxidation/phosphorylation coupling and moderately depolarized the mitochondrial membrane potential, resulting in a transient increase in the AMP/ATP ratio.
CONCLUSIONS:
Salidroside exerts an antidiabetic effect by improving the cellular metabolic flux through the activation of a mitochondria-related AMPK/PI3K/Akt/GSK3β pathway. | | J Surg Res. 2015 Jan 14. pii: S0022-4804(15)00057-8. | | Salidroside rescued mice from experimental sepsis through anti-inflammatory and anti-apoptosis effects.[Pubmed: 25676465] | Salidroside (SDS) is the main effective component of Rhodiola rosea L with a variety of pharmacologic properties. The objective of this study was to investigate the efficacy of SDS in the treatment of experimental sepsis in mice and explore the possible underlying action mechanisms.
METHODS AND RESULTS:
Sepsis was induced in C57BL/6 male mice via cecal ligation and puncture (CLP). The animals were divided into three groups as follows: sham, CLP, and CLP plus SDS. SDS (50 mg/kg) was injected intraperitoneally 1 h after operation. Postoperative survival of the mice, bacterial clearance in blood and peritoneal lavage fluid, cytokine secretion in blood, and histology of lung were evaluated. In addition, apoptosis of immune cells in the spleen and thymus were examined, respectively.
SDS administration prolonged the survival of the septic mice, inhibited the proinflammatory responses, and enhanced bacterial clearance. It also alleviated the pathologic changes in the lung and inhibited the apoptosis of immune cells in the spleen and thymus after CLP challenge.
CONCLUSIONS:
SDS exerts a protective effect in CLP-induced sepsis by attenuating the proinflammatory responses, enhancing bacterial clearance, and preserving adaptive immunity. SDS may be a promising therapeutic strategy for the treatment of sepsis. | | Behav. Brain Res., 2013, 244(244):70–81. | | Salidroside attenuates beta amyloid-induced cognitive deficits via modulating oxidative stress and inflammatory mediators in rat hippocampus.[Pubmed: 23396166] | Beta amyloid (Aβ)-induced oxidative stress and chronic inflammation in the brain are considered to be responsible for the pathogenesis of Alzheimer's disease (AD). Salidroside, the major active ingredient of Rhodiola crenulata, has been previously shown to have antioxidant and neuroprotective properties in vitro. The present study aimed to investigate the protective effects of Salidroside on Aβ-induced cognitive impairment in vivo.
METHODS AND RESULTS:
Rats received intrahippocampal Aβ1-40 injection were treated with Salidroside (25, 50 and 75 mg/kg p.o.) once daily for 21 days. Learning and memory performance were assessed in the Morris water maze (days 17-21). After behavioral testing, the rats were sacrificed and hippocampi were removed for biochemical assays (reactive oxygen species (ROS), superoxide dismutase (SOD), glutathione peroxidase (GPx), malondialdehyde (MDA), acetylcholinesterase (AChE), acetylcholine (ACh)) and molecular biological analysis (Cu/Zn-SOD, Mn-SOD, GPx, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, nuclear factor κB (NF-κB), inhibitor of κB-alpha (IκBα), cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), receptor for advanced glycation end products (RAGE)). Our results confirmed that Aβ1-40 peptide caused learning and memory deficits in rats. Further analysis demonstrated that the NADPH oxidase-mediated oxidative stress was increased in Aβ1-40-injected rats. Furthermore, NF-κB was demonstrated to be activated in Aβ1-40-injected rats, and the COX-2, iNOS and RAGE expression were also induced by Aβ1-40. However, Salidroside (50 and 75 mg/kg p.o.) reversed all the former alterations.
CONCLUSIONS:
Thus, the study indicates that Salidroside may have a protective effect against AD via modulating oxidative stress and inflammatory mediators. | | Evid Based Complement Alternat Med . 2017;2017:5398542. | | Salidroside Protects against MPP+-Induced Neuronal Injury through DJ-1-Nrf2 Antioxidant Pathway[Pubmed: 29234413] | | Abstract
Parkinson's disease (PD) is the second most common neurodegenerative disorder. We have found that Salidroside (Sal) exhibited neuroprotective effects against MPP+ toxicity. However, the molecular mechanism is not fully understood. In this study, we found that Sal significantly prevented MPP+-induced decrease of mRNA and protein expression of Nrf2, GCLc, SOD1, and SOD2 in SH-SY5Y cells. Moreover, silencing of Nrf2 significantly inhibited Sal-induced increase in mRNA and protein expression of GCLc, SOD1, and SOD2. But Nrf2 silence did not significantly impact Sal-exhibited effects on DJ-1 expression. Silencing of Nrf2 significantly suppressed the decrease of apoptosis induced by Sal in MPP+-treated SH-SY5Y cells. Sal significantly prevented MPP+-induced decrease of the mRNA and protein expression of DJ-1 in SH-SY5Y cells. Moreover, silencing of DJ-1 significantly inhibited Sal-induced increase in mRNA and protein expression of Nrf2, GCLc, SOD1, and SOD2 in MPP+-treated SH-SY5Y cells. These results indicated that DJ-1 was an upstream regulator of Nrf2 in the neuroprotective effects of Sal. Furthermore, silencing of DJ-1 significantly suppressed the decrease of apoptosis induced by Sal in MPP+-treated SH-SY5Y cells. In conclusion, Sal prevented MPP+-induced neurotoxicity through upregulation of DJ-1-Nrf2-antioxidant pathway. Our findings provide novel insights into the neuroprotective effects of Sal against PD. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)