| Description: |
Isofraxidin has definite anti-bacterial, anti-oxidant, analgesic,and anti-inflammatory activities, it inhibits expression of MMP-7 and in vitro cell invasion at a non-toxic level through inhibiting ERK1/2 phosphorylation in hepatoma cell lines.Isofraxidin is one possible radio-protector, it can protect leukemia cells from radiation-induced apoptosis via ROS/mitochondria pathway in a p53-independent manner. |
| In vitro: |
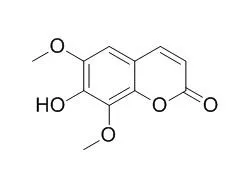
| Biol Pharm Bull. 2010;33(10):1716-22. | | Isofraxidin, a coumarin component from Acanthopanax senticosus, inhibits matrix metalloproteinase-7 expression and cell invasion of human hepatoma cells.[Pubmed: 20930381] | 7-Hydroxy-6,8-dimethoxy-2H-1-benzopyran-2-one (Isofraxidin) is a major coumarin component isolated from the stem bark of Acanthopanax senticosus, a widely used Chinese medicinal herb.
METHODS AND RESULTS:
We investigated Isofraxidin in its anti-tumor effects on human hepatoma cell lines HuH-7 and HepG2. Isofraxidin significantly inhibited hepatoma cell invasion, without affecting cell attachment or growth. Expression of 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced matrix metalloproteinase-7 (MMP-7) in hepatoma cells was inhibited by Isofraxidin at the both mRNA and protein levels. This inhibition tended to be greater in cells inoculated at low density than in those at high density. Isofraxidin showed an inhibitory effect on the phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) in hepatoma cells, whereas activator protein-1 (AP-1) DNA binding activity, nuclear factor-kappa B (NF-κB) nuclear translocation, and inhibitory kappa B (IκB) degradation were affected very little.
CONCLUSIONS:
These results indicate that Isofraxidin inhibits expression of MMP-7 and in vitro cell invasion at a non-toxic level through inhibiting ERK1/2 phosphorylation in hepatoma cell lines, which suggest Isofraxidin might become an effective agent for suppressing hepatoma cell invasion. |
|
| In vivo: |
| Immunobiology. 2015 Mar;220(3):406-13. | | Isofraxidin protects mice from LPS challenge by inhibiting pro-inflammatory cytokines and alleviating histopathological changes.[Pubmed: 25454811] | Isofraxidin (IF), the major bioactive component of Sarcandra glabra, has been reported to be an effective anti-inflammatory compound.
METHODS AND RESULTS:
In a previous study, we showed that Isofraxidin acts via the MAPK pathway to produce anti-inflammatory effects, both in vivo and in vitro. However, the effect and mechanism of action of Isofraxidin on inflammatory cytokines and NF-κB activation in vivo has not been investigated. We therefore aimed to evaluate how Isofraxidin regulates the production of inflammatory cytokines in vivo by intraperitoneal injection of Isofraxidin (1, 5 or 15mg/kg) prior to treatment with LPS (1mg/kg, i.p.). Macroscopic, biochemical and histopathological parameters were measured. Treatment with Isofraxidin prior to LPS challenge decreased mortality rate, body weight loss, organ coefficient and histopathological changes. Isofraxidin also suppressed the protein expression of NF-κB, levels of NO and IL-6 in serum and production of TNF-α in liver.
CONCLUSIONS:
Our results show that pretreatment with IF increases the survival rate following LPS stimulation in mice. The effect involves regulation of NF-κB signal which, in turn, regulates production of inflammatory cytokine TNF-α, suggesting that Isofraxidin may have a therapeutic effect against LPS-induced inflammatory disease. | | Int Immunopharmacol. 2012 Oct;14(2):164-71. | | Isofraxidin exhibited anti-inflammatory effects in vivo and inhibited TNF-α production in LPS-induced mouse peritoneal macrophages in vitro via the MAPK pathway.[Pubmed: 22800929] | Isofraxidin (IF) is a Coumarin compound that can be isolated from medicinal plants, such as Sarcandra glabra (Thunb.). Nakai is widely used in Asian countries for the treatment of anti-bacterial, anti-inflammatory and anti-tumour action.
METHODS AND RESULTS:
The present investigation was designed to evaluate the effect of Isofraxidin on inflammation and nociception. In addition, we investigated a potential novel mechanism to explain the anti-inflammatory properties of Isofraxidin. In vivo, xylene-induced mouse ear edema, carrageenan-induced rat paw edema, LPS-induced mouse endotoxic shock, acetic acid-induced mice writhing and formalin-induced mouse pain models were used to evaluate the anti-inflammatory activity of Isofraxidin. In vitro, we examined the effects of Isofraxidin inhibition on TNF-α production and the regulation of ERK1/2 and p38 phosphorylation activity in LPS-induced mouse peritoneal macrophages. Our results demonstrated that Isofraxidin can significantly decrease xylene-induced ear edema, carrageenan-induced paw edema, acetic acid-induced writhing and formalin-induced pain. Moreover, Isofraxidin greatly inhibited the production of TNF-α in the serum of LPS-stimulated mice and peritoneal macrophages, and it decreased phospho-p38 and ERK1/2 protein expression in LPS-stimulated mouse peritoneal macrophages.
CONCLUSIONS:
Overall, our data suggest that Isofraxidin possesses significant analgesic and anti-inflammatory activities that may be mediated through the regulation of pro-inflammatory cytokines, TNF-α and the phosphorylation of p38 and ERK1/2. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)