| In vitro: |
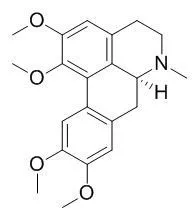
| Biochem Pharmacol. 2013 Nov 15;86(10):1497-506. | | Studies on the in vivo contribution of human cytochrome P450s to the hepatic metabolism of glaucine, a new drug of abuse.[Pubmed: 23988488] | Glaucine ((S)-5,6,6a,7-tetrahydro-1,2,9,10-tetramethoxy-6-methyl-4H-dibenzo [de,g]quinoline), main isoquinoline alkaloid of Glaucium flavum (Papaveraceae), is used as antitussive, but also as recreational drug of abuse. Glaucine was mainly metabolized by O- and N-demethylation to four isomers in rats. So far, only scarce pharmacokinetic data were available. Therefore, the aim of the presented study was to assess the involvement of the ten most important cytochrome P450 (P450) isoforms in the main metabolic steps and determination of their kinetic parameters using the metabolite formation approach.
METHODS AND RESULTS:
Reference standards of investigated metabolites were synthesized for quantification. In addition, the impact of isomeric standards was tested for calibration and the use of simple peak area ratios on the kinetic constants and resulting contribution of P450 isoforms on estimated hepatic clearance. Kinetic profiles of all metabolite formations followed classic Michaelis-Menten behavior. Km values were between 25 and 140μM, Vmax between 0.10 and 1.92pmol/min/pmol. Using the relative activity factor approach, the hepatic clearance was calculated to be 27 and 73% for 2-O-demethylation by CYP1A2 and CYP3A4, 82, 3, and 15% for 9-O-demethylation by CYP1A2, CYP2C19, and CYP2D6, and finally <1 and 99% for N-demethylation by CYP2D6 and CYP3A4.
CONCLUSIONS:
These data were confirmed by inhibition tests. The calibration mode for determination of the metabolite concentrations had no relevant impact on the estimation of in vivo hepatic clearance of Glaucine. As Glaucine was metabolized via three initial steps and different P450 isoforms were involved in the hepatic clearance of Glaucine, a clinically relevant interaction with single inhibitors should not be expected. | | Bioorg Med Chem. 2008 Aug 1;16(15):7457-61. | | Cinnamoyl- and hydroxycinnamoyl amides of glaucine and their antioxidative and antiviral activities.[Pubmed: 18590964 ] | The aporphine alkaloid Glaucine has been converted into 3-aminomethylGlaucine and its free amino group has been linked to cinnamic, ferulic, sinapic, o-, and p-coumaric acids.
METHODS AND RESULTS:
The antioxidative potential of the synthesized amides was studied against DPPH(*) test. All of the tested compounds demonstrated higher radical scavenging activity than Glaucine and 3-aminomethylGlaucine, and lower antioxidative effect than the free hydroxycinnamic acids. The newly synthesized compounds were tested in vitro for antiviral activity against viruses belonging to different taxonomic groups. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)