| In vitro: |
| J. Wood Sci., 2009, 55(4):308-13. | | Antimicrobial activity against Streptococcus sobrinus and glucosyltransferase inhibitory activity of taxifolin and some flavanonol rhamnosides from kempas (Koompassia malaccensis) extracts[Reference: WebLink] | Twenty plant materials collected from the islands of Java and Kalimantan in Indonesia were extracted with 50% aqueous ethanol (crude extract).
METHODS AND RESULTS:
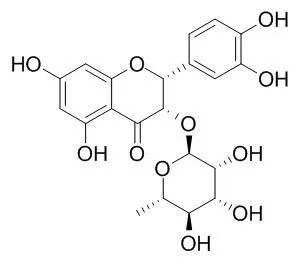
The crude extracts were assayed for antimicrobial activities against Streptococcus sobrinus and for glucosyltransferase (GTase) inhibition. Fourteen extracts inhibited the growth of S. sobrinus by more than 50% and six extracts inhibited GTase activity by more than 50% at a concentration of 100 μg/ml. Koompassia malaccensis (kempas) extracts showed 90% depression of S. sobrinus growth and 80% inhibition of GTase activity at a concentration of 100 μg/ml. Kempas crude extracts were subjected to column chromatography using Sephadex LH-20 and then preparative high-performance liquid chromatography to isolate four compounds A, B, C, and D. These compounds were identified as taxifolin and the flavanonol rhamnoside isomers neoastilbin, astilbin, and Isoastilbin, respectively, from 1H and 13C nuclear magnetic resonance (NMR) spectra and other two-dimensional NMR techniques (COSY, HMBC, and HMQC). Each compound depressed the growth of S. sobrinus over a concentration range of 9.3242.7 μg/ml and showed GTase inhibitory activity with IC₅₀ values in the range 27.4-57.3 μg/ml.
CONCLUSIONS:
Taxifolin and flavanonol rhamnoside isomers isolated for the first time from kempas could be potent compounds for preventing dental caries. | | Wood Research Journal ,2010, 1(1):45-9. | | Anti-acne and Tyrosinase Inhibition Properties of Taxifolin and Some Flavanonol Rhamnosides from Kempas ( Koompassia malaccensis )[Reference: WebLink] | Taxifolin (1) and some flavanonol rhamnosides (neoastilbin (2), astilbin (3), and Isoastilbin (4)) have been isolated from kempas (Koompassia malaccensis). Our previous research about antimicrobial activity against Streptococcus sobrinus and glucosyltransferase inhibitory activity of these compounds have been reported.
METHODS AND RESULTS:
Now, we carried out the antiacne and tyrosinase inhibition properties of all four compounds. Antimicrobial against Propionibacterium acnes, P. acnes lipase inhibitory activity and antioxidant activity were established for anti-acne activity. Tyrosinase inhibition property was measured using L-tyrosine and L-DOPA as substrate. The results for anti-acne showed that no antimicrobial activity against P. acnes for all compounds, the best lipase inhibition properties showed on compound 4 with IC50 about 1.36 g/ml, and % inhibition for antioxidant at concentration 10 g/ml are 31.16, 25.64, 28.47, and 31.01% respectively. Tyrosinase inhibition of compound 1 at concentration 1 mg/ml is 24.12% for monophenolase and 5.18% for diphenolase. Compound 2 has tyrosinase inhibition about 25.95% (monophenolase) and 14.18% (diphenolase) at concentration 1 mg/ml. Compound 3 has tyrosinase inhibition about 27.17% (monophenolase) and 6.23% (diphenolase) at same concentration, while compound 4 has tyrosinase inhibition about 11.17% (monophenolase) and 9.75% (diphenolase). |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)