| In vitro: |
| Mol Pharmacol. 2005 Dec;68(6):1534-42. | | Stereospecific induction of nuclear factor-kappaB activation by isochamaejasmin.[Pubmed: 16141313] | The root of Stellera chamaejasme L. is a traditional Chinese herb termed Rui Xiang Lang Du and has been used to treat solid tumors, tuberculosis and psoriasis. Exactly how S. chamaejasme L. regulates cellular responses remains unclear.
METHODS AND RESULTS:
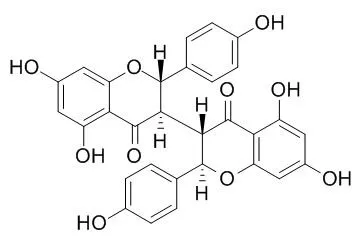
We examined four biflavonoids isolated from S. chamaejasme L., including isochamaejasmin(Isochamaejasmine), two of its stereo-isomers and a methyl derivative, in functional assays originally designed to screen ligands for the G protein-coupled formyl peptide receptor-like 1 (FPRL1). Isochamaejasmin(Isochamaejasmine) was found to induce the expression of a nuclear factor (NF)-kappaB-directed reporter gene in transfected HeLa cells with an EC50 of 3.23 microM, independently of FPRL1. The isochamaejasmin-stimulated NF-kappaB reporter activity was accompanied by nuclear translocation of NF-kappaB proteins and was blocked by a dominant-negative construct of IkappaBalpha. Isochamaejasmin(Isochamaejasmine) also induced time-dependent phosphorylation of the mitogen-activated protein kinases extracellular signal-regulated kinase 1/2 and p38, and a novel protein kinase C (PKCdelta). Likewise, inhibition of these kinases with the respective pharmacological inhibitors significantly reduced the isochamaejasmin(Isochamaejasmine)-stimulated NF-kappaB activation. It is noteworthy that the two stereoisomers and the methyl derivative did not induce detectable activation of NF-kappaB and were more cytotoxic than isochamaejasmin, which could partially rescue cycloheximide-induced apoptosis. Inhibition of NF-kappaB activation reversed the anti-apoptotic effect of isochamaejasmin(Isochamaejasmine).
CONCLUSIONS:
These results provide the first evidence for a potential mechanism of action by S. chamaejasme L., and indicate that structurally similar compounds derived from S. chamaejasme L. may have different pharmacological properties. | | Phytochemistry. 2010 May;71(7):785-91. | | Antiplasmodial activity of (I-3,II-3)-biflavonoids and other constituents from Ormocarpum kirkii.[Pubmed: 20189612 ] | Preliminary screening of a series of medicinal plants, traditionally used in Tanzania, showed an IC(50) of 15.6-31.2 microg/ml for the crude extract of the root of Ormocarpum kirkii S. Moore (Papilionaceae) against Plasmodium falciparum. A bioguided isolation was performed in order to isolate the active constituents.
METHODS AND RESULTS:
Twelve constituents were obtained and identified using NMR and MS data, and optical rotation measurements. The compounds comprised seven (I-3,II-3)-biflavonoids, three (I-3,II-3)-bi-4-phenyldihydrocoumarins, an isoflavanone and a C-glucosylated flavone. Six compounds, liquiritigeninyl-(I-3,II-3)-naringenin, apigeninyl-(I-3,II-3)-naringenin, 7-O-beta-D-glucopyranosylchamaejasmin, (3R,4S,3''R,4''S)-5,5''-di-O-methyldiphysin, 7-O-beta-D-glucopyranosyldiphysin, and 4''-hydroxydiphysolone, were isolated in addition to six known components. The compounds were evaluated for antimicrobial activity in a broad screening panel, including P. falciparum. Seven of these showed antiplasmodial activity; isochamaejasmin(Isochamaejasmine) being the most active with an IC(50) of 7.3+/-3.8 microM, but the selectivity was rather limited.
CONCLUSIONS:
Thus, these constituents may contribute, at least in part, to the antimalarial use of O. kirkii in traditional medicine. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)