| In vitro: |
| Planta Med. 2006 Apr;72(5):424-9. | | Induction of apoptosis by isoflavonoids from the leaves of Millettia taiwaniana in human leukemia HL-60 cells.[Pubmed: 16557456] |
METHODS AND RESULTS:
We have isolated two new isoflavonoids, millewanin-F (1) and furowanin-A (2), together with five known isoflavonoids from the leaves of Millettia taiwaniana Hayata (Leguminosae) and examined their effects on the growth of human leukemia HL-60 cells. Among the isolated isoflavonoids, furowanin-A (2), warangalone (3), isoerysenegalensein-E (4), and euchrenone b10 (6) showed significant cytotoxicity against HL-60 cells. After treatment with three of the cytotoxic isoflavonoids, furowanin-A (2), warangalone (3), and Isoerysenegalensein E (4), fluorescence microscopy with Hoechst 33,342 staining revealed that the percentage of apoptotic cells with fragmented nuclei and condensed chromatin increased in a time-dependent manner. In addition, the activities of caspase-9 and caspase-3 were also enhanced in a time-dependent manner upon treatment with the isoflavonoids 2, 3, and Isoerysenegalensein E. Caspase-9 and caspase-3 inhibitors suppressed apoptosis induced by isoflavonoids 2, 3, and Isoerysenegalensein E.
CONCLUSIONS:
These results suggest that the isoflavonoids induced apoptosis in HL-60 cells through activation of the caspase-9/caspase-3 pathway, which is triggered by mitochondrial dysfunction. | | Journal of Health Science, 2006 , 52 (2) :186-191. | | AntiEstrogenic Activity of Prenylated Isoflavones from Millettia pachycarpa: Implications for Pharmacophores and Unique Mechanisms[Reference: WebLink] | Phytoestrogens containing isoflavonoids are thought to exhibit preventative effects on estrogen-responsive diseases. Chemical modifications, such as prenylation, in biosynthetic processes enhance the structural variety of isoflavonoids and prompted us to carry out a structure-activity relationship study.
METHODS AND RESULTS:
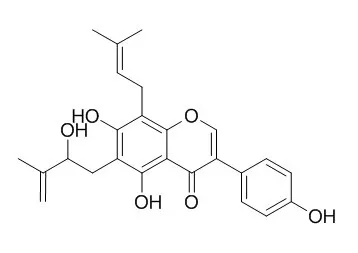
We determined the estrogenic/anti-estrogenic activities and estrogen receptor (ER)-binding affinities of eight kinds of prenylated isoflavones isolated from Millettia pachycarpa (Leguminosae), and those of two kinds of non-prenylated compounds (genistein and daidzein). By comparing these compounds, the pharmacophores for estrogenic/anti-estrogenic activities were elucidated. None of the tested compounds (except genistein) were estrogenic on ligand-dependent yeast-two hybrid assay. On the other hand, 5 isoflavones showed distinct anti-estrogenic activity. Unexpectedly, the most potent antagonists, Isoerysenegalensein E and 6,8-diprenylorobol, showed anti-estrogenic activity comparable to that of 4-hydroxytamoxifen, a typical ER antagonist.
CONCLUSIONS:
This suggests that genistein became an antagonist after prenylation and hydroxylation. The pharmacophores providing genistein with strong anti-estrogenic activity were as follows: prenyl groups of the 6- and 8-positions on the A-ring, hydroxyl group of the 6-prenyl moiety or the B-ring (catechol form), non-cyclization of the prenyl group with the A-ring, and non-hydroxylation of the 8-prenyl group on the A-ring. The ER-binding affinities of the isoflavonoids were not sufficiently high to explain their potent antagonistic activities, thus suggesting 17β-estradiol-non-competitive mechanisms. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)