| Cell Research: |
| Br J Haematol. 2006 Mar;132(5):615-22. | | Oxindole alkaloids from Uncaria tomentosa induce apoptosis in proliferating, G0/G1-arrested and bcl-2-expressing acute lymphoblastic leukaemia cells.[Pubmed: 16445836] | Natural products are still an untapped source of promising lead compounds for the generation of antineoplastic drugs.
METHODS AND RESULTS:
Here, we investigated for the first time the antiproliferative and apoptotic effects of highly purified oxindole alkaloids, namely isopteropodine (A1), pteropodine (A2), Isomitraphylline (A3), uncarine F (A4) and mitraphylline (A5) obtained from Uncaria tomentosa, a South American Rubiaceae, on human lymphoblastic leukaemia T cells (CCRF-CEM-C7H2). Four of the five tested alkaloids inhibited proliferation of acute lymphoblastic leukaemia cells. Furthermore, the antiproliferative effect of the most potent alkaloids pteropodine (A2) and uncarine F (A4) correlated with induction of apoptosis. After 48 h, 100 micromol/l A2 or A4 increased apoptotic cells by 57%. CEM-C7H2 sublines with tetracycline-regulated expression of bcl-2, p16ink4A or constitutively expressing the cowpox virus protein crm-A were used for further studies of the apoptosis-inducing properties of these alkaloids. Neither overexpression of bcl-2 or crm-A nor cell-cycle arrest in G0/G1 phase by tetracycline-regulated expression of p16INK4A could prevent alkaloid-induced apoptosis.
CONCLUSIONS:
Our results show the strong apoptotic effects of pteropodine and uncarine F on acute leukaemic lymphoblasts and recommend the alkaloids for further studies in xenograft models. |
|
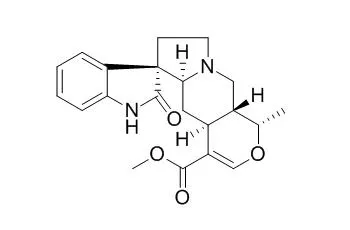
| Structure Identification: |
| Nat Prod Commun. 2009 Jul;4(7):907-10. | | Phytochemical characterization of the leaves of Mitragyna speciosa grown in U.S.A.[Pubmed: 19731590] | Mitragyna speciosa (Rubiaceae) has traditionally been used in the tropical regions of Asia, Africa and Indonesia as a substitute for opium. Indole alkaloids are the most common compounds that have been isolated.
METHODS AND RESULTS:
We investigated the constituents of the leaves of M. speciosa that was grown at the University of Mississippi. Several alkaloids were isolated, including ajmalicine, corynantheidine, Isomitraphylline, mitraphylline, paynantheine, isocorynantheidine, 7-hydroxymitragynine and mitragynine, but their percentages were lower than those in a commercial Thai sample of "kratom". In addition, we isolated the flavonoid epicatechin, a saponin daucosterol, the triterpenoid saponins quinovic acid 3-O-beta-D-quinovopyranoside, quinovic acid 3-O-beta-D-glucopyranoside, as well as several glycoside derivatives including 1-O-feruloyl-beta-D-glucopyranoside, benzyl-beta-D-glucopyranoside, 3-oxo-alpha-ionyl-O-beta-D-glucopyranoside, roseoside, vogeloside, and epivogeloside.
CONCLUSIONS:
This is the first report of the last group of compounds having been isolated from a Mitragyna species. Biological studies are currently underway to test these compounds for opioid activity. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)