| Kinase Assay: |
| Thromb Res. 2013 Jul;132(1):e58-63. | | Down-regulation of endothelial protein C receptor shedding by persicarin and isorhamnetin-3-O-galactoside.[Pubmed: 23726966 ] | Increasing evidence has shown that beyond its role in coagulation, endothelial protein C receptor (EPCR) plays an important role in the cytoprotective pathway.
Previous reports have shown that EPCR can be shed from the cell surface, and that this is mediated by tumor necrosis factor-α converting enzyme (TACE) and that sEPCR levels are increased in patients with systemic inflammatory diseases. Persicarin and Isorhamnetin 3-O-galactoside (I3G) are active compounds from Oenanthe javanica, which has been widely studied for its neuroprotective, antioxidant, and barrier protective activities. However, little is known of the effects of persicarin on EPCR shedding.
METHODS AND RESULTS:
Here, we investigated this issue by monitoring the effects of persicarin and I3G on phorbol-12-myristate 13-acetate (PMA) and on cecal ligation and puncture (CLP)-mediated EPCR shedding and underlying mechanisms. According to the results, persicarin and I3G induced potent inhibition of PMA and CLP-induced EPCR shedding by suppressing expression of TACE. In addition, persicarin and I3G reduced PMA-stimulated phosphorylation of p38MAPK, extracellular regulated kinases (ERK) 1/2, and c-Jun N-terminal kinase (JNK).
CONCLUSIONS:
Given these results, persicarin and I3G could be used as a candidate therapeutic for treatment of severe vascular inflammatory diseases. | | Arch Pharm Res. 2011 Sep;34(9):1561-9. | | Inhibition of 5-lipoxygenase and skin inflammation by the aerial parts of Artemisia capillaris and its constituents.[Pubmed: 21975819] | The aerial parts of Artemisia capillaris Thunberg (Compositae) have been used in Chinese medicine as a liver protective agent, diuretic, and for amelioration of skin inflammatory conditions. This study was conducted to establish the scientific rationale for treating skin inflammation and to find active principles from A. capillaris.
METHODS AND RESULTS:
To accomplish these goals, the 70% ethanol extract of the aerial parts of A. capillaris (AR) was prepared and its 5-lipoxygenase (5-LOX) inhibitory action was studied since 5-LOX products are known to be involved in several allergic and skin inflammatory disorders. AR showed potent inhibitory activity against 5-LOX-catalyzed leukotriene production by ionophore-induced rat basophilic leukemia-1 cells, with an IC(50) of < 1.0 μg/mL. Nine major compounds, scopoletin, scopolin, scoparone, esculetin, quercetin, capillarisin, isorhamnetin, 3-O-robinobioside, Isorhamnetin 3-O-galactoside and chlorogenic acid, were isolated from A. capillaris, and their effects were examined to identify the active principle(s). Several coumarin and flavonoid derivatives were found to be 5-LOX inhibitors. In particular, esculetin and quercetin were potent inhibitors, with IC(50) values of 6.6 and 0.7 μM, respectively. Against arachidonic acid-induced ear edema in mice, AR, and esculetin strongly inhibited edematic response. AR and esculetin also inhibited delayed-type hypersensitivity response in mice.
CONCLUSIONS:
In conclusion, AR and some of their major constituents are 5-LOX inhibitors, and these in vitro and in vivo activities may contribute to the therapeutic potential of AR in skin inflammatory disorders in traditional medicine. |
|
| Structure Identification: |
| Afr J Tradit Complement Altern Med. 2013 Nov 2;11(1):67-72. | | The flavonoid constitunts of Leucaena leucocephala. Growing in Egypt, and their biological activity.[Pubmed: 24653555] | Leucaena leucocephala is native to Southern Mexico and Northern Central America, but is now naturalized throughout the tropics. The phyto-chemical data of L. leucocephala revealed the presence of terpenes, flavonoids, coumarins and sterols. Various parts of L. leucocephala have been reported to have medicinal properties.
METHODS AND RESULTS:
Flavonoids were isolated from the aerial parts of L. leucocephala. Antioxidant activity of the extracts and the isolated compounds was evaluated using (DPPH), as well as their cytotoxic activity using a single tumor [Ehrlish ascites carcinoma cells].
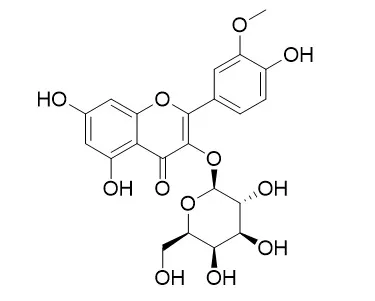
The flavonoidal constituents isolated from chloroform, ethyl acetate and n-butanol fractions of the aqueous alcoholic extract of aerial parts of Leucaena leucocephala were identified as Caffeic acid, Isorhamnetin, Chrysoeriol, Isorhamnetin 3-O-galactoside, Kaempferol-3-O-rubinoside, Quercetin-3-O-rhamnoside and Luteolin-7-glucoside. Chemical structures of the isolated compounds were identified by TLC, PC and spectral techniques (UV, (1)H-NMR and MS). The ethyl acetate fraction and the isolated flavonoidal compounds showed high antioxidant activity compared to Trolox (standard antioxidant compound). The different fractions and isolated compounds of Leucaena leucocephala exhibited no cytotoxic activity against Ehrlich-ascitis carcinoma cell line at the tested concentrations.
CONCLUSIONS:
This is the first record of the flavonoids in the aerial parts of Leucaena leucocephala (L.) except Quercetin-3-O-rhamnoside. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)