| In vitro: |
| Eur J Med Chem. 2015 Aug 1;102:256-265. | | Synthesis, cytotoxicity and inhibition of NO production of ivangustin enantiomer analogues.[Pubmed: 26280922] |
METHODS AND RESULTS:
The eight novel Ivangustin enantiomer analogues possessing α-methylene-γ-butyrolactone moiety have been synthesized using (4S6R, 4S6S)-4-tert-butyldimethylsilyloxy-6-methylcyclohex-2-en-1-one (1) as starting material. These transformations were mainly carried out by aldol condensation reaction and one-pot annelation procedure. The stereochemistry of these synthesized analogues was determined by NOE analysis. Their cytoxicity was evaluated against the human cancer cell lines HCT-116 (colon), HL-60 (leukemia), QGY-7701 (liver), SMMC-7721 (liver), A549 (lung), MCF-7 (breast). The results showed that these analogues were more selective against the cell lines HL-60 and QGY-7701. Analogue 17 exhibited potent cytotoxicity and high selectivity toward HL-60 cell line with IC50 value of 1.02 μM, which suggested that it might be a promising anti-cancer lead compound. The inhibitory activities against NO production and the cytotoxicities in RAW 264.7 macrophages were determined at the same time.
CONCLUSIONS:
All of the analogues significantly inhibited the NO production with IC50 value in the range of 3.44-6.99 μM. Analogues 17, 22, 23 and 7 showed higher cytotoxicities, indicated their inhibitory activities against NO production may be influenced by the cytotoxicities. | | Nat. Prod. Commun.,2016,11(1):7-10. | | Cytotoxic and Pro-apoptotic Activities of Sesquiterpene Lactones from Inula britannica.[Pubmed: 26996005] |
METHODS AND RESULTS:
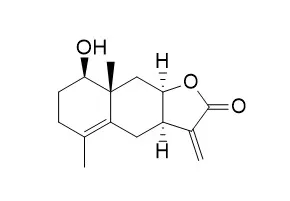
In this study, five known sesquiterpene lactones (STL) with an α-methylene-γ-lactone motif, including two eudesmanolides, 1β-hydroxyalantolactone (1) and Ivangustin (2), and three 1,10-seco-eudesmanolides, 1-O-acetylbritannilactone (3), 1,6-O,O-diacetylbritannilactone (4), and 6α-O-(2- methylbutyryl)britannilactone (5) were isolated from the flower heads of the medicinal plant Inula britannica. Their structures were characterized by spectroscopic methods. X-ray data of 2 is reported for the first time. Among them, eudesmanolides 1 and 2 exhibited remarkable cytotoxicity against HEp2, SGC-7901 and HCT116 human cancer cell lines, comparable with etoposide (Vp-16) used as reference drug. Furthermore, treatment of HEp2 cells with 1 induced apoptosis associated with cleaved procaspase-3 and PARP.
CONCLUSIONS:
The biological assays carried out with normal cells (CHO) revealed that all sesquiterpenes were weakly selective against the cancer cell lines tested. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)