| In vitro: |
| Brain Research, 1982, 237(1):248-253. | | L-aspartic acid induces a region of negative slope conductance in the current-voltage relationship of cultured spinal cord neurons.[Reference: WebLink] |
Individual murine spinal cord neurons were grown in dissociated tissue cultures.
METHODS AND RESULTS:
Using either one or two conventional intracellular microelectrodes neurons were voltage-clamped and current-voltage (I–V) curves were constructed using command pulses.L-Aspartic acid was then applied and I–V curves repeated in the presence of this excitatory amino acid.L-Aspartic acid induced a region of negative slope conductance (RNSC) in the steady-state I–V relationships of these neurons.
CONCLUSIONS:
Such a RNSC accounts for the apparent reduction of conductance observed in response to excitatory amino acids5,7,8 and its instability likely contributes to the electrogenesis of bursting in central neurons. | | Enzyme & Microbial Technology, 2000, 27( 1–2):19-25. | | Selective production of L-aspartic acid and L-phenylalanine by coupling reactions of aspartase and aminotransferase in Escherichia coli.[Reference: WebLink] |
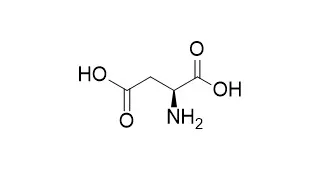
METHODS AND RESULTS:
With l-aspartate (L-Asp) as the amino donor, l-phenylalanine (L-Phe) can be prepared from phenylpyruvate (PPA) via an amination reaction mediated by aminotransferase (encoded by aspC). On the other hand, L-Asp can be produced by an aspartase (encoded by aspA) -catalyzed reaction using fumaric acid as substrate. To overproduce aspartase in Escherichia coli, the aspA gene was cloned and overexpressed 180 times over the wild-type level. The use of AspA-overproducing E. coli strain for L-Asp production exhibited an 83% conversion, approaching to the theoretical yield, whereas the wild-type strain obtained scarcely L-Asp. Furthermore, the recombinant strain overproducing both AspA and AspC was able to produce L-Asp and L-Phe simultaneously by using fumaric acid and PPA as substrates. As a result, the conversion yields obtained for L-Asp and L-Phe were 78% and 85%, respectively. In sharp contrast, the wild-type strain attained a conversion of L-Phe less than 15% and an undetectable level of L-Asp.
CONCLUSIONS:
This result illustrates a potential and attractive process to yield both L-Asp and L-Phe by coupling AspA and AspC. A further study on the repeated use of the recombinant strain immobilized with calcium alginate showed that after eight batch runs L-Asp conversion maintained roughly constant (around 75%), whereas L-Phe conversion dropped to 65% from 81%. This result indicates the stability of AspA being superior to AspC. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)