| In vitro: |
| J Nat Med. 2011 Jan;65(1):237-40. | | Cytotoxic and EGFR tyrosine kinase inhibitory activities of aglycone derivatives obtained by enzymatic hydrolysis of oleoside-type secoiridoid glucosides, oleuropein and ligustroside.[Pubmed: 21042869] |
METHODS AND RESULTS:
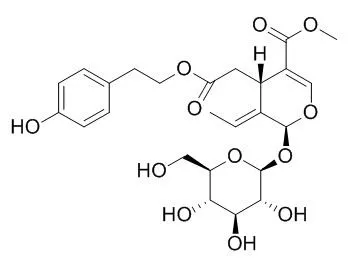
Hydrolysis of oleoside-type secoiridoid glucosides, oleuropein (1) and Ligustroside (2), in the presence of β-glucosidase provided their aglycones, named (5S,8R,9S)-7-3,4-dihydroxyphenethyl elenolate (3) and (5S,8R,9S)-7-4-hydroxyphenethyl elenolate (4), respectively. The structures of 3 and 4 were identified by spectroscopic means and optical rotation measurements.
CONCLUSIONS:
Evaluation of the cytotoxic and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitory activities of compounds 1-4 showed that compounds 3 and 4 exhibited moderate cytotoxicity against a disease-oriented panel of 39 human cancer cell lines in vitro, whereas compound 3 inhibited the enzyme. | | J Chromatogr A. 2006 Apr 21;1112(1-2):311-8. | | Isolation and identification of radical scavengers in olive tree (Olea europaea) wood.[Pubmed: 16426626 ] | Several extracts of Olea europaea wood (Picual olive cultivar) were obtained with solvents of different polarity and their antioxidant activities determined.
METHODS AND RESULTS:
The active compounds were detected in fractions of an ethyl acetate extract using HPLC with on-line radical scavenging detection. After applying different separation techniques, hydroxytyrosol, tyrosol, cycloolivil, 7-deoxyloganic acid, oleuropein and Ligustroside were isolated and characterized. Hydroxytyrosol showed a higher activity than the natural antioxidant rosmarinic acid in scavenging the DPPH model radical.
CONCLUSIONS:
Cycloolivil and oleuropein showed stronger activities than the synthetic antioxidant BHT against the same radical. Ligustroside, tyrosol and 7-deoxyloganic acid showed little activity. The latter compound has not been previously identified in the genus Olea. | | Chem Pharm Bull (Tokyo). 2001 Nov;49(11):1471-3. | | In vitro evaluation of secoiridoid glucosides from the fruits of Ligustrum lucidum as antiviral agents.[Pubmed: 11724241] | Six secoiridoid glucosides, lucidumoside C (1), oleoside dimethylester (2), neonuezhenide (3), oleuropein (4), Ligustroside (5) and lucidumoside A (6), isolated from the fruits of Ligustrum lucidum (Oleaceae), were examined in vitro for their activities against four strains of pathogenic viruses, namely herpes simplex type I virus (HSV-1), influenza type A virus (Flu A), respiratory syncytial virus (RSV) and parainfluenza type 3 virus (Para 3).
METHODS AND RESULTS:
Antiviral activities were evaluated by the cytopathic effect (CPE) inhibitory assay. The purpose was to check if the antioxidative potency of these glucosides correlated with their antiviral potency. Results showed that none of the glucosides had any significant activity against HSV-1 and Flu A. Oleuropein, however, showed significant antiviral activities against RSV and Para 3 with IC50 value of 23.4 and 11.7 microg/ml, respectively. Lucidumoside C, oleoside dimethylester and Ligustroside showed potent or moderate antiviral activities against Para 3 with IC50 values of 15.6-20.8 microg/ml.
CONCLUSIONS:
These results also documented that the anti-oxidative potency of these secoiriodoid glucosides was not directly related to their antiviral effects. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)