| In vitro: |
| Antiviral Res. 2010 Mar;85(3):490-5. | | Inhibition of the Epstein-Barr virus lytic cycle by moronic acid.[Pubmed: 19969023] | Epstein-Barr virus (EBV) expresses two transcription factors, Rta and Zta, during the immediate-early stage of the lytic cycle to activate the transcription of viral lytic genes.
METHODS AND RESULTS:
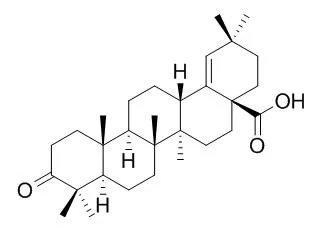
Our immunoblotting and flow cytometry analyses find that Moronic acid, found in galls of Rhus chinensis and Brazilian propolis, at 10microM inhibits the expression of Rta, Zta, and an EBV early protein, EA-D, after lytic induction with sodium butyrate. This study also finds that Moronic acids inhibits the capacity of Rta to activate a promoter that contains an Rta-response element, indicating that Moronic acid interferes with the function of Rta. On the other hand, Moronic acid does not appear to influence with the transactivation function of Zta. Therefore, the lack of expression of Zta and EA-D after Moronic acid treatment is attributable to the inhibition of the transactivation functions of Rta. Because the expression of Zta, EA-D and many EBV lytic genes depends on Rta, the treatment of P3HR1 cells with Moronic acid substantially reduces the numbers of EBV particles produced by the cells after lytic induction.
CONCLUSIONS:
This study suggests that Moronic acid is a new structural lead for anti-EBV drug development. | | J Med Chem. 2010 Apr 22;53(8):3133-41. | | Anti-AIDS agents 81. Design, synthesis, and structure-activity relationship study of betulinic acid and moronic acid derivatives as potent HIV maturation inhibitors.[Pubmed: 20329730] |
METHODS AND RESULTS:
In our continuing study of triterpene derivatives as potent anti-HIV agents, different C-3 conformationally restricted betulinic acid (BA, 1) derivatives were designed and synthesized in order to explore the conformational space of the C-3 pharmacophore. 3-O-Monomethylsuccinyl-betulinic acid (MSB) analogues were also designed to better understand the contribution of the C-3' dimethyl group of bevirimat (2), the first-in-class HIV maturation inhibitor, which is currently in phase IIb clinical trials. In addition, another triterpene skeleton, Moronic acid (MA, 3), was also employed to study the influence of the backbone and the C-3 modification toward the anti-HIV activity of this compound class.
CONCLUSIONS:
This study enabled us to better understand the structure-activity relationships (SAR) of triterpene-derived anti-HIV agents and led to the design and synthesis of compound 12 (EC(50): 0.0006 microM), which displayed slightly better activity than 2 as a HIV-1 maturation inhibitor. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)