| Structure Identification: |
| Journal of organic chemistry, 2003, 34(49):6279. | | The enantiospecific, stereospecific total synthesis of the ring-A oxygenated sarpagine indole alkaloids (+)-majvinine, (+)-10-methoxyaffinisine, and (+)-N(a)-methylsarpagine, as well as the total synthesis of the alstonia bisindole alkaloid macralstonidine.[Pubmed: 12895062 ] |
METHODS AND RESULTS:
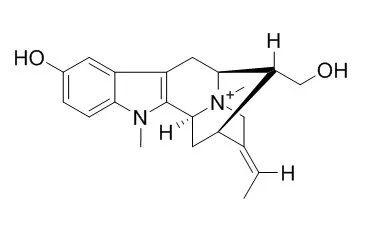
The first stereospecific, enantiospecific total synthesis of the ring-A oxygenated sarpagine indole alkaloids (+)-N(a)-methylsarpagine (N-Methylsarpagine methosalt, 8), (+)-majvinine (14), and (+)-10-methoxyaffinisine (49), as well as the first total synthesis of the Alstonia bisindole alkaloid macralstonidine (9), has been accomplished. This approach employed the Schöllkopf chiral auxiliary for the stereospecific construction of the desired d-(+)-tryptophan unit required for the asymmetric Pictet-Spengler reaction. In addition, the strategy was doubly convergent for the enolate-mediated Pd(0) coupling process and the asymmetric Pictet-Spengler reaction can be employed to synthesize both macroline (2) and N(a)-methylsarpagine (8), the coupling of which provides macralstonidine (9).
CONCLUSIONS:
This approach to ring-A substituted alkoxyindole alkaloids should find wide application for the synthesis of other alkaloids for it is stereospecific and either enantiomer can be prepared with ease. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)