| Kinase Assay: |
| Food Funct. 2019 Sep 1;10(9):6062-6073. | | Natural alkaloids from lotus plumule ameliorate lipopolysaccharide-induced depression-like behavior: integrating network pharmacology and molecular mechanism evaluation.[Pubmed: 31486445 ] | Depression is a mental disorder that brings severe burdens to patients and their families. Neuroinflammation and neurotrophins are involved in depression. Lotus plumule is a nutritional food with medicinal values.
METHODS AND RESULTS:
In the present study, we tried to clarify the anti-depressive effect and molecular mechanism of lotus plumule. Network pharmacological analysis, behavior tests, qRT-PCR and western blotting were used. We found 7 potential active components and 91 targets from the TCMSP database. KEGG analysis suggested that lotus plumule significantly affected nitrogen metabolism, calcium signaling, and inflammatory mediator regulation signaling pathways. Consistent with those effects, total alkaloids of lotus plumule (TLA) and active alkaloids differently suppressed the nitric oxide (NO) production and pro-inflammatory mediators. TLA and Higenamine significantly ameliorated LPS-induced depression-like behavior, increased BDNF levels, suppressed microglia activation, and inhibited the expression of ER stress-related proteins. Meanwhile, TLA and Higenamine activated microglia autophagy by increasing the beclin-1 and LC3B-II expression. Additionally, in the presence of autophagy inhibitor 3-MA, TLA and Higenamine did not reduce the LPS-induced NO production or pro-inflammatory mediators.
CONCLUSIONS:
Collectively, TLA and Higenamine attenuated LPS-induced depression-like behavior by regulating BDNF-mediated ER stress and autophagy. Therefore, drinking tea of lotus plumule may provide a potential strategy for preventing depression.
| | Oncol Rep. 2018 Oct;40(4):2127-2136. | | Higenamine enhances the antitumor effects of cucurbitacin B in breast cancer by inhibiting the interaction of AKT and CDK2.[Pubmed: 30106443 ] | Cucurbitacin B (Cu B), a tetracyclic triterpenoid derived from Trichosanthes kirilowii Maxim, exhibits anticancer effects against various types of tumor. Higenamine, isolated from Radix Aconiti Lateralis Preparata, has been used as a dietary supplement for regulating metabolic function.
METHODS AND RESULTS:
The present study suggested that Higenamine enhances Cu B-induced cytotoxicity in breast cancer cells and in vivo. Network pharmacology analysis was used to identify the possible mechanism of action. Cu B alone inhibited breast cancer cell growth, induced apoptosis, and arrested the cell cycle in the G2/M phase. Cu B combined with Higenamine potentiated the cytotoxic effect of Cu B, resulting in the enhanced induction of apoptosis and G2/M arrest. The network pharmacology analysis also found that the major predicted targets of Cu B in breast cancer were AKT, endoplasmic reticulum, farnesyltransferase, CAAX box, α, platelet-derived growth factor receptor α, peroxisome proliferator-activated receptor, RET proto-oncogene, and vascular endothelial growth factor A. The possible targets of Higenamine involved in the synergic action were cyclin A2, cyclin-dependent kinase 2 (CDK2), dihydrofolate reductase, and protein kinase CAMP‑activated catalytic subunit α.
CONCLUSIONS:
The associated pathways were summarized by Kyoto Encyclopedia of Genes and Genomes pathway analysis, and it was hypothesized that Higenamine may enhance the antitumor effects of Cu B in breast cancer through inhibition of the interaction of AKT and CDK2. The protein expression was assayed by western blot analysis. The combined treatment also resulted in significant inhibition of growth in vivo. |
|
| Cell Research: |
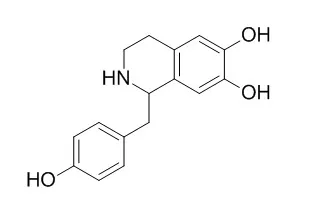
| Biomed Pharmacother. 2019 Jul;115:108881. | | Protective effects of higenamine combined with [6]-gingerol against doxorubicin-induced mitochondrial dysfunction and toxicity in H9c2 cells and potential mechanisms.[Pubmed: 31028997 ] | Higenamine (HG) is a well-known selective activator of beta2-adrenergic receptor (β2-AR) with a positive inotropic effect. The present study showed that HG combined with [6]-gingerol (HG/[6]-GR) protects H9c2 cells from doxorubicin (DOX)-induced mitochondrial energy metabolism disorder and respiratory dysfunction.
METHODS AND RESULTS:
H9c2 cells were pretreated with HG/[6]-GR for 2 h before DOX treatment in all procedures. Cell viability was quantified by a cell counting kit‑8 assay. Cardiomyocyte morphology, proliferation, and mitochondrial function were detected by a high content screening (HCS) assay. Cell mitochondrial stress was measured by a Seahorse XFp analyzer. To further investigate the protective mechanism of HG/[6]-GR, mRNA and protein expression levels of PPARα/PGC-1α/Sirt3 pathway-related molecules were detected. The present data demonstrated that protective effects of HG/[6]-GR combination were presented in mitochondria, which increased cell viability, ameliorated DOX-induced mitochondrial dysfunction, increased mitochondrial oxygen consumption rate (OCR) and extracellular acidification rate (ECAR). Most importantly, the protective effects were abrogated by GW6471 (a PPARα inhibitor) and ameliorated by Wy14643 (a PPARα agonist). Moreover, the combined use of HG and [6]-GR exerted more profound protective effects than either drug as a single agent.
CONCLUSIONS:
In conclusion, the results suggested that HG/[6]-GR ameliorates DOX-induced mitochondrial energy metabolism disorder and respiratory function impairment in H9c2 cells, and it indicated that the protective mechanism may be related to upregulation of the PPARα/PGC-1α/Sirt3 pathway, which promotes mitochondrial energy metabolism and protects against heart failure. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)