| In vitro: |
| Yao Xue Xue Bao. 2015 May;50(5):579-82. | | Chemical constituents from Morus notabilis and their cytotoxic effect.[Pubmed: 26234140] |
METHODS AND RESULTS:
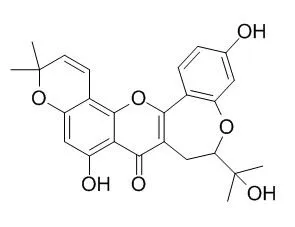
Une new flavonoids named as notabilisin K (1), together with tour known compounds, morusin (2), mulberrofuran A (3), Neocyclomorusin (4) and mornigrol F (5) are separated from 95% ethanol extracts of the twigs of Morus notabilis. Compounds 2-5 are separated from this plant for the first time. Notabilisin I, notabilisin J exhibits certain effect against cells of HCT-116, HepG2 and A2780 with IC50 values ranging from 1.47 μmol x L(-1) to 5.46 μmol x L(-1).
CONCLUSIONS:
Morusin exhibits strong effect against five kinds of human cancer cells (BGC823, A2780, HCT-116, HepG2 and NCI-H1650) with IC50 values ranging from 0.74 μmol x L(-1) to 1.58 μmol x L(-1). | | Springerplus. 2015 Dec 30;4:823. | | Antibacterial activity of nineteen selected natural products against multi-drug resistant Gram-negative phenotypes.[Pubmed: 26753111 ] | The present study was designed to assess the antimicrobial activity of 19 natural products belonging to terpenoids, alkaloids, thiophenes and phenolics against a panel of 14 Gram-negative multidrug-resistant (MDR) bacteria.
METHODS AND RESULTS:
The results demonstrated that amongst the studied compounds, alkaloids and terpenoids were less active contrary to flavonoids: Neocyclomorusin (3) and candidone (6) and isoflavonoids: neobavaisoflavone (8) and daidzein (12). Thiophene, 2-(penta-1,3-diynyl)-5-(3,4-dihydroxybut-1-ynyl)thiophene (17) showed moderate and selective activities. Compounds 3, 6, 8 and 12 displayed minimal inhibitory concentration (MIC) ranged from 4 to 256 μg/mL on all the 14 tested bacteria. MIC values below 10 μg/mL were obtained with 8, 3, 6 and 12 against 50, 42.9, 35.7 and 21.4 % of the tested bacteria. The lowest MIC value of 4 μg/mL was obtained with compound 3 against Klebsiella pneumoniae ATCC11296, Enterobacter cloacae BM47, compound 6 against Escherichia coli ATCC8739, K. pneumoniae ATCC11296, E. cloacae BM47 and compound 8 against K. pneumoniae ATCC11296 and E. cloacae BM47. The activity of flavonoid 3 was better or equal to that of chloramphenicol in all tested K. pneumoniae, Providencia stuartii, E. aerogenes, E. cloacae and Pseudomonas aeruginosa strains. Within isoflavonoids, neobavaisoflavone scaffold was detected as a pharmacophoric moiety.
CONCLUSIONS:
This study indicates that natural products such as 3, 6 and 8 could be explored more to develop antimicrobial drugs to fight MDR bacterial infections. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)