| Kinase Assay: |
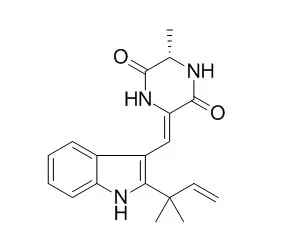
| Neurotoxicology. 2013 Mar;35:30-40. | | Neoechinulin A suppresses amyloid-β oligomer-induced microglia activation and thereby protects PC-12 cells from inflammation-mediated toxicity.[Pubmed: 23261590] | Focus was given to evaluate the ability of Neoechinulin A, an indole alkaloid isolated from marine-derived Microsporum sp., to attenuate microglial activation by oligomeric amyloid-β 1-42 (Aβ42).
METHODS AND RESULTS:
Neoechinulin A treatment significantly inhibited the generation of reactive oxygen and nitrogen species in Aβ42-activated BV-2 microglia cells. In addition, we found that Neoechinulin A significantly suppressed the production of neurotoxic inflammatory mediator tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and prostaglandin E2 (PGE2) in activated BV-2 cells. Moreover, the treatment downregulated the protein and gene expressions of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), TNF-α, IL-1β and IL-6. Further, activated microglia-mediated apoptosis of PC-12 pheochromocytoma cells was significantly repressed by Neoechinulin A. The molecular mechanism studies suggested that Neoechinulin A may block the phosphorylation of mitogen-activated protein kinase (MAPK) molecule p38, apoptosis signal-regulating kinase 1 (ASK-1) and nuclear translocation of nuclear factor-κB (NF-κB) p65 and p50 subunits. Regulation of these signalling pathways have most probably contributed to the anti-inflammatory activity of Neoechinulin A.
CONCLUSIONS:
Collectively, these results suggest that with further studies Neoechinulin A have a potential to be developed as a modulator of neuroinflammatory process in AD. | | Biol Pharm Bull. 2011;34(2):243-8. | | Neoechinulin a impedes the progression of rotenone-induced cytotoxicity in PC12 cells.[Pubmed: 21415535] | Neoechinulin A, an indole alkaloid from marine fungi, can protect PC12 cells from the cytotoxicity of 1-methyl-4-phenylpyridinium (MPP(+)), a Parkinson disease-inducing neurotoxin, by ameliorating downstream events resulting from mitochondrial complex I inactivation. However, the cytoprotective mechanisms remained unclear.
METHODS AND RESULTS:
In this study, by using rotenone, another parkinsonian-inducing neurotoxin targeting mitochondrial complex I, we investigated the cytoprotective mechanism of Neoechinulin A. Rotenone-induced cell death was associated with accelerated glucose consumption, and excess glucose supplementation in the culture medium almost completely suppressed cell death, suggesting that glucose deficiency in the medium is critical for triggering cell death in this model. Co-treatment with Neoechinulin A, but not Neoechinulin A pre-treatment before rotenone exposure, significantly impeded cell death by rotenone. Although the presence of Neoechinulin A did not affect the accelerated glycolytic turnover in rotenone-treated cells, it paradoxically decreased ATP levels in the cells, suggesting increased ATP consumption.
CONCLUSIONS:
Although the link between the decreased ATP levels and cytoprotection is not clear at present, it suggests that Neoechinulin A may ameliorate rotenone toxicity by activating a cytoprotective machinery that requires ATP. |
|
| Cell Research: |
| Biol Pharm Bull. 2012;35(7):1105-17. | | Neoechinulin a imparts resistance to acute nitrosative stress in PC12 cells: a potential link of an elevated cellular reserve capacity for pyridine nucleotide redox turnover with cytoprotection.[Pubmed: 22791159] | Treatment of PC12 cells with fungus-derived alkaloid Neoechinulin A for more than 12 h renders the cells resistant to subsequent superoxide (O₂⁻)/nitric oxide (NO) insults derived from 3-morpholinosydnonimine (SIN-1). However, the underlying mechanism(s) remains largely unclear. To elucidate the mechanism(s), we assessed the specificity of the cytoprotection afforded by Neoechinulin A treatment using other cytocidal stressors and also clarified the resulting cellular alterations, focusing on the antioxidant and metabolic enzymes systems.
METHODS AND RESULTS:
Neoechinulin A treatment for more than 12 h endowed PC12 cells with significant resistance to transient NO toxicity, but not persistent NO toxicity, bolus H₂O₂ toxicity, or oxidative insult from the redox cycling quinone menadione. Cellular antioxidant system profiling revealed no substantial potentiation of the activity of any antioxidant enzyme in lysate from the Neoechinulin A-treated cells excluding glutathione (GSH) content, which was significantly decreased (>50%), resulting in a proportional compromise in the thiol-reducing activity of the intact cells. In addition, no differences were observed in the activity for any nicotinamide adenine dinucleotide (phosphate) reduced form (NAD(P)H)-generating enzyme, steady-state NAD(P)H/nicotinamide adenine dinucleotide (phosphate) oxidized form (NAD(P)⁺) ratios, or the levels of total NAD(P)H. Nevertheless, the Neoechinulin A-treated intact cells exhibited increased NAD(P)H redox turnover when driven by extracellular tetrazolium. The structurally inactive analog preechinulin failed to protect cells against NO toxicity or induce these alterations, suggesting their link with the cytoprotective mechanism.
CONCLUSIONS:
These results suggest that Neoechinulin A, despite disabling the GSH defense system, confers cytoprotection against nitrosative stresses by elevating the cellular reserve capacity for NAD(P)H generation, which could offset crippling of energy-supplying systems due to nitrosative stress. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)