| In vitro: |
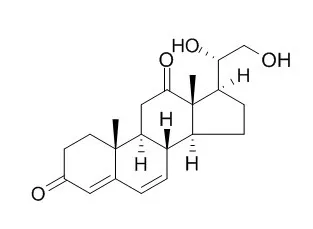
| J Nat Prod. 2007 Jan;70(1):14-8. | | Bioactive pregnanes from Nerium oleander.[Pubmed: 17253842] | A new cholesterol derivative, pentalinonsterol (cholest-4,20,24-trien-3-one, 1), and a new polyoxygenated pregnane sterol glycoside, pentalinonside (2), together with 18 known compounds, including 14 sterols (3-16), three coumarins (17-19), and a triterpene (20), were isolated from a n-hexane partition of a methanol extract of the roots of the Mexican medicinal plant Pentalinon andrieuxii.
METHODS AND RESULTS:
Structure elucidation of compounds 1 and 2 was accomplished by spectroscopic data interpretation. All isolates were evaluated in vitro for their antileishmanial activity. Among these compounds, 6,7-dihydroneridienone (15) was found to be the most potent principle against promastigotes of Leishmania mexicana (L. mexicana). The cholesterol analogue, pentalinonsterol (1), together with two known sterols, 24-methylcholest-4,24(28)-dien-3-one (3) and neridienone (16), also exhibited significant leishmanicidal activity in this same bioassay. Compounds 1, 3, 15, 16, cholest-4-en-3-one (4), and cholest-5,20,24-trien-3β-ol (7), showed strong antileishmanial activity against amastigotes of L. mexicana, and 4 was found to be the most potent agent with an IC(50) value of 0.03μM. All the isolates were also evaluated for their cytotoxicity in non-infected bone marrow-derived macrophages, but none of these compounds was found active towards this cell line.
CONCLUSIONS:
The intracellular parasites treated with compounds 1, 3, 4, 15, and 16 were further studied by electron microscopy; morphological abnormalities and destruction of the amastigotes were observed, as a result of treatment with these compounds. | | Phytochemistry. 2012 Oct;82:128-35. | | Sterols with antileishmanial activity isolated from the roots of Pentalinon andrieuxii.[Pubmed: 22840389] | A new cholesterol derivative, pentalinonsterol (cholest-4,20,24-trien-3-one, 1), and a new polyoxygenated pregnane sterol glycoside, pentalinonside (2), together with 18 known compounds, including 14 sterols (3-16), three coumarins (17-19), and a triterpene (20), were isolated from a n-hexane partition of a methanol extract of the roots of the Mexican medicinal plant Pentalinon andrieuxii.
METHODS AND RESULTS:
Structure elucidation of compounds 1 and 2 was accomplished by spectroscopic data interpretation. All isolates were evaluated in vitro for their antileishmanial activity. Among these compounds, 6,7-dihydroneridienone (15) was found to be the most potent principle against promastigotes of Leishmania mexicana (L. mexicana). The cholesterol analogue, pentalinonsterol (1), together with two known sterols, 24-methylcholest-4,24(28)-dien-3-one (3) and neridienone (16), also exhibited significant leishmanicidal activity in this same bioassay. Compounds 1, 3, 15, 16, cholest-4-en-3-one (4), and cholest-5,20,24-trien-3β-ol (7), showed strong antileishmanial activity against amastigotes of L. mexicana, and 4 was found to be the most potent agent with an IC(50) value of 0.03μM. All the isolates were also evaluated for their cytotoxicity in non-infected bone marrow-derived macrophages, but none of these compounds was found active towards this cell line.
CONCLUSIONS:
The intracellular parasites treated with compounds 1, 3, 4, 15, and 16 were further studied by electron microscopy; morphological abnormalities and destruction of the amastigotes were observed, as a result of treatment with these compounds. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)