| Description: |
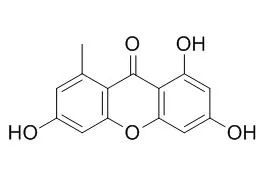
Norlichexanthone has the potential to treat and/or prevent lifestyle-related diseases, including metabolic syndrome, type 2 diabetes, atherosclerosis and cardiovascular diseases.Norlichexanthone has antibacterial and antimalarial activities, it shows strong activity against Bacillus subtilis with IC50 in the range of 1-5uM, it also significantly inhibits the growth of methicillin-resistant Staphylococcus aureus with IC50 of 20.95±1.56uM. |
| In vitro: |
| Med Chem. 2011 Jul;7(4):250-6. | | Norlichexanthone isolated from fungus P16 promotes the secretion and expression of adiponectin in cultured ST-13 adipocytes.[Pubmed: 21568875] |
METHODS AND RESULTS:
We found that the butanol extract of a fungus P16 culture extract possessed such an activity, and isolated Norlichexanthone as an active compound through activity-guided fractionation. Oil red O staining showed that Norlichexanthone induced adipogenesis in ST-13 cells. Its differentiation-inducing activity was supported by the observation that Norlichexanthone dose-dependently increased the mRNA expression of fatty acid-binding protein and peroxisome proliferator activated receptor γ (PPARγ), markers of adipocyte differentiation. Western blot analysis demonstrated that the compound enhanced the secretion of adiponectin protein in a dose-dependent manner. An increase in mRNA expression of adiponectin was also observed in the Norlichexanthone-treated ST-13 cells. Actinomycin D treatment blocked the enhancement of adiponectin mRNA expression by Norlichexanthone, suggesting that it is the result of increased transcription. A luciferase reporter assay indicated that Norlichexanthone was unlikely to be an agonist of PPARγ, implying that its action of mechanism might differ from those of thiazolidinediones which upregulate adiponectin expression via activation of PPARγ.
CONCLUSIONS:
These findings suggest the possibility that Norlichexanthone has the potential to treat and/or prevent lifestyle-related diseases, including metabolic syndrome, type 2 diabetes, atherosclerosis and cardiovascular diseases. | | Appl Environ Microbiol. 2015 May 29. | | SnPKS19 encodes the polyketide synthase for alternariol mycotoxin biosynthesis in the wheat pathogen Parastagonospora nodorum.[Pubmed: 26025896] | Alternariol (AOH) is an important mycotoxin from the Alternaria fungi. AOH was detected for the first time in the wheat pathogen Parastagonospora nodorum in a recent study.
METHODS AND RESULTS:
Here, we exploited reverse genetics to demonstrate that SNOG_15829 (SnPKS19), a close homolog of Penicillium aethiopicum Norlichexanthone (NLX) synthase gene gsfA, is required for AOH production. We further validate that SnPKS19 is solely responsible for AOH production by heterologous expression in Aspergillus nidulans. The expression profile of SnPKS19 based on previous P. nodorum microarray data correlated with the presence of AOH in vitro and its absence in planta. Subsequent characterization of the ΔSnPKS19 mutants showed that SnPKS19 and AOH are not involved in virulence and oxidative stress tolerance. Identification and characterization of the P. nodorum SnPKS19 cast light on a possible alternative AOH synthase gene in Alternaria alternata and allowed us to survey the distribution of AOH synthase genes in other fungal genomes. We further demonstrate that phylogenetic analysis could be used to differentiate between AOH synthases and the closely related NLX synthases.
CONCLUSIONS:
This study provides the basis for studying the genetic regulation of AOH production and for development of molecular diagnostic methods for detecting AOH-producing fungi in the future. | | Fitoterapia. 2012 Jan;83(1):209-14. | | Polyketides with antimicrobial activity from the solid culture of an endolichenic fungus Ulocladium sp.[Pubmed: 22061662] |
METHODS AND RESULTS:
Two new polyketides, 7-hydroxy-3, 5-dimethyl-isochromen-1-one (1) and 6-hydroxy-8-methoxy-3a-methyl-3a,9b-dihydro-3H-furo[3,2-c]isochromene-2,5-dione (2), along with eleven known compounds, 5'-methoxy-6-methyl-biphenyl-3,4,3'-triol (3), 7-hydroxy-3-(2-hydroxy-propyl)-5-methyl-isochromen-1-one (4), rubralactone (5), isoaltenuene (6), altenuene (7), dihydroaltenuenes A (8), altenusin (9), alterlactone (10), 6-O-methylNorlichexanthone (11), Norlichexanthone (12), and griseoxanthone C (13) were isolated from the culture of the endolichenic fungus Ulocladium sp. Compound 2 was obtained as a racemate with an unprecedented chemical skeleton. The NMR data assignments for 3 and 4 were achieved for the first time. Compounds 1-13 were screened for their antimicrobial and radical scavenging activities.
CONCLUSIONS:
Compound 1 showed some antifungal activity against Candida albicans SC 5314 with IC(50) of 97.93 ± 1.12 μM. Compounds 11-13 showed strong activity against Bacillus subtilis with IC(50) in the range of 1-5 μM. Compound 12 significantly inhibited the growth of methicillin-resistant Staphylococcus aureus with IC(50) of 20.95 ± 1.56 μM. Compounds 9 and 10 showed strong radical scavenging activity in comparison with vitamin C. The plausible biosynthetic pathways for compounds 1, 2, and 4-8 were discussed. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)