| Description: |
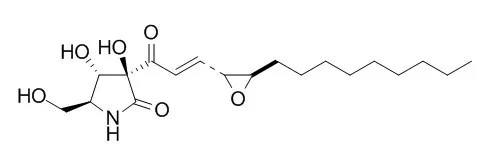
Pramanicin, an antimicrobial agent, has vasorelaxant effect, it induces a slow endothelium-dependent relaxation, which could be reversed with the NO synthase inhibitor, L-NOARG, it has potent, selective, and irreversible inhibitory effect on the endothelial.Pramanicin as a potential apoptosis-inducing small molecule, which acts through a well-defined JNK- and p38-dependent apoptosis signalling pathway in Jurkat T leukemia cells. |
| In vitro: |
| J Org Chem. 2015 Mar 6;80(5):2661-75. | | Synthesis of mimics of pramanicin from pyroglutamic acid and their antibacterial activity.[Pubmed: 25647715] | Epoxypyrrolidinones are available by epoxidation of carboxamide-activated bicyclic lactam substrates derived from pyroglutamate using aqueous hydrogen peroxide and tertiary amine catalysis.
METHODS AND RESULTS:
In the case of an activating Weinreb carboxamide, further chemoselective elaboration leads to the efficient formation of libraries of epoxyketones. Deprotection may be achieved under acidic conditions to give epoxypyroglutaminols, although the ease of this process can be ameliorated by the presence of internal hydrogen bonding. Bioassay against S. aureus and E. coli indicated that some compounds exhibit antibacterial activity.
CONCLUSIONS:
These libraries may be considered to be structural mimics of the natural products Pramanicin and epolactaene. More generally, this outcome suggests that interrogation of bioactive natural products is likely to permit the identification of "privileged" structural scaffolds, providing frameworks suitable for optimization in a short series of chemical steps that may accelerate the discovery of new antibiotic chemotypes. Further optimization of such systems may permit the rapid identification of novel systems suitable for antibacterial drug development. | | Vascul Pharmacol. 2003 Jan;40(1):35-42. | | Pramanicin, an antifungal agent, raises cytosolic Ca2+ and causes cell death in vascular endothelial cells.[Pubmed: 12646408] | The effects of a newly discovered antifungal agent, Pramanicin, on cytosolic Ca(2+) and cell viability of cultured bovine pulmonary artery endothelial cells and on endothelium-dependent relaxation of dog carotid arterial rings were investigated by digital dynamic fluorescence ratio imaging and morphological and contractility studies, respectively.
METHODS AND RESULTS:
Pramanicin 100 microM, previously shown to cause maximal endothelium-dependent and NO-mediated vascular relaxation, induced a small transient elevation of cytosolic Ca(2+) concentration in Ca(2+)-free medium; subsequent introduction of 1 mM Ca(2+) caused a steady, nonsaturating increase of Ca(2+), which could be brought down to the basal level by the addition of EGTA. At the single cell level, the elevation of cytosolic Ca(2+) initiates from the cell periphery and progresses toward the central region. When added to the plateau phase of phenylephrine-induced contraction, Pramanicin induced a slow endothelium-dependent relaxation, which could be reversed with the NO synthase inhibitor, L-NOARG. When preincubated with vascular tissue, Pramanicin resulted in an irreversible loss of endothelial function characterized by the lack of carbachol-induced relaxation. Pramanicin caused cell injury characterized by plasmalemmal bleb formation, leading to cell death characterized by Trypan blue staining of the nuclei in cultured vascular endothelial cells in a concentration- and time-dependent manner. Such Pramanicin-induced cell death was not associated with Ca(2+)-mediated or NO-mediated mechanisms. The time course of Ca(2+) elevation corresponds with that of Pramanicin-induced relaxation of precontracted arterial rings, whereas the time course of endothelial cell death corresponds to that of Pramanicin-induced loss of endothelial function as assessed by carbachol-induced relaxation.
CONCLUSIONS:
The Pramanicin analogue, PMC-A, a by-product of the biosynthesis of Pramanicin, in which the epoxy group is replaced by a CC bond, caused little endothelial-dependent relaxation, but it was able to cause endothelial cell dysfunction, albeit to a lesser extent compared to Pramanicin, suggesting a role of the epoxy group in Pramanicin for its vasorelaxant effect. | | J Pharmacol Sci. 2003 Jul;92(3):203-8. | | The epoxy group of pramanicin is required for the optimal endothelium-dependent relaxation of rat aorta.[Pubmed: 12890885] | The vascular effects of a newly discovered anti-fungal agent, Pramanicin (PMC), and its two analogues, PMC-A, in which the epoxy group is replaced by a - HC = CH - bond, and PMC-B, on which the diene is converted to the saturated (CH(2))(4)-derivative, respectively, were investigated in rat aorta.
METHODS AND RESULTS:
All three compounds caused an initial endothelial-dependent relaxation, which is prevented either by removal of endothelium or inclusion of the nitric oxide synthase inhibitor L-NAME. Upon prolonged incubation with the aortic rings, they also caused endothelial cell dysfunction characterized as reduced relaxation to carbachol (CCh). These effects were the strongest for PMC, being completely inhibitory at 20 microM after 30 min incubation, whereas those of PMC-A and PMC-B were smaller and comparable with each other, causing 30 - 40% inhibition at 20 micro M. PMC and its analogues had no effect on KCl-induced contraction and also had no effect on relaxation induced by sodium nitroprusside, suggesting that these compounds had no effect on the basic mechanisms of the contractile elements. Phenylephrine (PE)-induced contraction, however, was significantly reduced in the presence of these compounds, the inhibitory effect being strongest with PMC, but this inhibitory action was rapidly reversible and not of the competitive mode with respect to PE.
CONCLUSIONS:
We conclude that the epoxy group in PMC is required for the optimal vascular effects. We have discussed and speculated upon the possible mechanisms of action of PMC. The potent, selective, and irreversible inhibitory effect of PMC on the endothelial function points to its potential development into an anti-angiogenic drug. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)