| In vitro: |
| J Ethnopharmacol. 2013 Jan 9;145(1):381-5. | | New antiplasmodial alkaloids from Stephania rotunda.[Pubmed: 23127648] |
METHODS AND RESULTS:
A new aporphine alkaloid named vireakine (2) along with two known alkaloids stephanine (1) and Pseudopalmatine (8), described for the first time in Stephania rotunda, and together five known alkaloids tetrahydropalmatine (3), xylopinine (4), roemerine (5), cepharanthine (6) and palmatine (7) were isolated and identified. The structure of the new alkaloid was established on the basis of 1D and 2D NMR experiments and mass spectrometry. The compounds were evaluated for their in vitro antiplasmodial and cytotoxic activities.
CONCLUSIONS:
All tested compounds showed significant antiplasmodial activities with IC(50) ranged from 1.2 μM to 52.3 μM with a good selectivity index for Pseudopalmatine with IC(50) of 2.8 μM against W2 strain of Plasmodium falciparum and IC(50)>25 μM on K562S cells. | | J Nat Prod. 2010 Oct 22;73(10):1632-5. | | Characterization of Acetylcholinesterase Inhibitory Constituents from Annona glabra Assisted by HPLC Microfractionation.[Pubmed: 20828184] |
METHODS AND RESULTS:
The active fraction of the EtOH extract of the stem of Annona glabra against acetylcholinesterase (AChE) was analyzed by combining HPLC microfractionation with a bioassay. The analytical-scale sample was fractionated by HPLC-DAD into 96-well microplates, which, after evaporation, were assayed against AChE. The active subfractions were scaled up by separation over semipreparative HPLC to give 20 compounds. Four of these, (7S,14S)-(-)-N-methyl-10-O-demethylxylopinine salt (3), S-(-)-7,8-didehydro-10-O-demethylxylopininium salt (10), S-(-)-7,8-didehydrocorydalminium salt (11), and 5-O-methylmarcanine D (17), were assigned as new natural products. In addition, compounds 10 and 11 represent the first natural occurrence of 7,8-didehydroprotoberberines.
CONCLUSIONS:
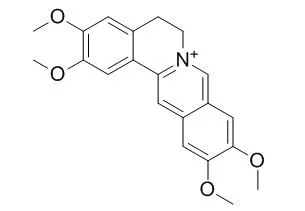
Compound 3, pseudocolumbamine (12), palmatine (15), and Pseudopalmatine (16) showed anti-AChE IC50 values of 8.4, 5.0, 0.4, and 1.8 μM, respectively. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)