| Kinase Assay: |
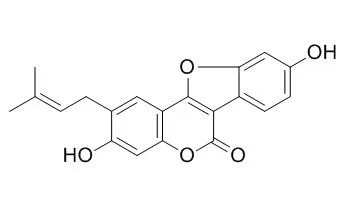
| Oncol Lett. 2016 Aug;12(2):971-976. | | Psoralidin inhibits proliferation and enhances apoptosis of human esophageal carcinoma cells via NF-κB and PI3K/Akt signaling pathways.[Pubmed: 27446379] | Esophageal cancer is the most common gastrointestinal cancer. Psoralidin exhibits antioxidant, anti-apoptotic, anti-inflammatory and antitumor effects, which result in the inhibition of cancer formation.
METHODS AND RESULTS:
The present study aimed to investigate the effect of Psoralidin on esophageal carcinoma proliferation and growth, and to elucidate its underlying mechanism of action. The effect of Psoralidin on cell proliferation was investigated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Using an annexin V-fluorescein isothiocyanate/propidium iodide apoptosis detection kit and 4',6-diamidino-2-phenylindole staining assay, the present study demonstrated that Psoralidin significantly enhanced apoptosis of human esophageal carcinoma Eca9706 cells. In addition, caspase-3 activity was analyzed with a caspase-3 colorimetric assay kit, while nuclear factor (NF)-κB activity and protein phosphatidylinositol 3-kinase (PI3K)/Akt expression were measured with an NF-κB enzyme-linked immunosorbent assay kit and western blot analysis, respectively. Eca9706 cells were treated with a PI3K agonist in order to investigate the mechanism of action of Psoralidin. It was observed that Psoralidin was able to decrease the proliferation and promote the cellular apoptosis of Eca9706 cells in a dose-dependent manner. Furthermore, Psoralidin was also able to inhibit the caspase-3 activity of Eca9706 cells in a dose-dependent manner. In addition, Psoralidin inhibited NF-κB activity and reduced PI3K and Akt protein expression in Eca9706 cells. Notably, the PI3K agonist was able to reverse the effect of Psoralidin on Eca9706 cells.
CONCLUSIONS:
The results of the present study demonstrated that Psoralidin was able to inhibit proliferation and enhance apoptosis of human esophageal carcinoma cells via the NF-κB and PI3K/Akt signaling pathways. | | Bioorg Med Chem Lett. 2014 Mar 1;24(5):1403-6. | | Psoralidin, a coumestan analogue, as a novel potent estrogen receptor signaling molecule isolated from Psoralea corylifolia.[Pubmed: 24507928] | A novel biological activity of Psoralidin as an agonist for both estrogen receptor (ER)α and ERβ agonist has been demonstrated in our study.
METHODS AND RESULTS:
Psoralidin has been characterized as a full ER agonist, which activates the classical ER-signaling pathway in both ER-positive human breast and endometrial cell lines as well as non-human cultured cells transiently expressing either ERα or ERβ. The estrogenic activity was determined using the relative expression levels of either reporter or the endogenous genes dependent on the agonist-bound ER to the estrogen response element (ERE). Psoralidin at 10 μM was able to induce the maximum reporter gene expression corresponding to that of E2-treated cells and such activation of the ERE-reporter gene by Psoralidin was completely abolished by the cotreatment of a pure ER antagonist, implying that the biological activities of Psoralidin are mediated by ER. Psoralidin was also able to induce the endogenous estrogen-responsive gene, pS2, in human breast cancer cells MCF-7. It was observed that activation of the classical ER-signaling pathway by Psoralidin is mediated via induction of ER conformation by Psoralidin and direct binding of the Psoralidin-ER complex to the EREs present in the promoter region of estrogen-responsive genes, as shown by chromatin immunoprecipitation assay results.
CONCLUSIONS:
Finally, molecular docking of Psoralidin to the ligand binding pocket of the ERα showed that Psoralidin is able to mimic the binding interactions of E2, and thus, it could act as an ER agonist in the cellular environment. | | Eur J Pharmacol. 2011 Jan 10;650(1):102-9. | | Psoralidin inhibits LPS-induced iNOS expression via repressing Syk-mediated activation of PI3K-IKK-IκB signaling pathways.[Pubmed: 20951127] | Psoralidin has been reported to inhibit lipopolysaccharide (LPS)-induced nitric oxide (NO) production, but the mechanisms of the action remain unclear. Thus, the impact of Psoralidin on signaling pathways known to be implicated in NO synthesis was explored in LPS-activated RAW264.7 macrophages by using RT-PCR and Western blotting. Consistent with NO inhibition, Psoralidin suppressed LPS-induced expression of inducible NO synthase (iNOS) by abolishing IκB kinase (IKK) phosphorylation, IκB degradation and nuclear factor κB (NF-κB) nuclear translocation without effecting mitogen-activated protein kinases (MAPKs) phosphorylation. Exposure to wortmannin abrogated IKK/IκB/NF-κB-mediated iNOS expression, suggesting activation of such a signal pathway might also be phosphoinositide-3-kinase (PI3K) dependent. By using Src inhibitor PP2, Janus kinase 2 (JAK-2) inhibitor AG490, Bruton's tyrosine kinase (Btk) inhibitor LFM-A13 and spleen tyrosine kinase (Syk) inhibitor piceatannol, the results showed that piceatannol clearly repressed NO production more potently than the other inhibitors. Furthermore, piceatannol significantly repressed LPS-induced PI3K/Akt phosphorylation and the downstream IKK/IκB activation, suggesting that Syk is an upstream key regulator in the activation of PI3K/Akt-mediated signaling. In fact, transfection with siRNA targeting Syk obviously reduced iNOS expression. Interestingly, LPS-induced phosphorylations of Syk and PI3K-p85 were both significantly blunted by Psoralidin treatment.
CONCLUSIONS:
The present results show that interfering with Syk-mediated PI3K phosphorylation might contribute to the NO inhibitory effect of Psoralidin via blocking IKK/IκB signaling propagation in LPS-stimulated RAW 264.7 macrophages. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)