| Kinase Assay: |
| Biochem Biophys Res Commun. 2016 Apr 29;473(2):415-20. | | Pterosin B has multiple targets in gluconeogenic programs, including coenzyme Q in RORα-SRC2 signaling.[Pubmed: 26970301] | Hepatic gluconeogenic programs are regulated by a variety of signaling cascades. Glucagon-cAMP signaling is the main initiator of the gluconeogenic programs, including glucose-6-phosphatase catalytic subunit (G6pc) gene expression.
METHODS AND RESULTS:
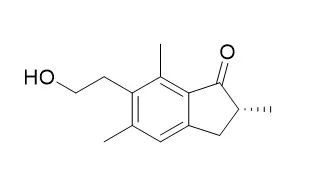
Pterosin B, an ingredient in Pteridium aquilinum, inhibits salt-inducible kinase 3 signaling that represses cAMP-response element-binding protein regulated transcription coactivator 2, an inducer of gluconeogenic programs. As the results, Pterosin B promotes G6pc expression even in the absence of cAMP. In this work, however, we noticed that once cAMP signaling was initiated, Pterosin B became a strong repressor of G6pc expression. The search for associated transcription factors for Pterosin B actions revealed that retinoic acid receptor-related orphan receptor alpha-steroid receptor coactivator 2 (RORα-SRC2) complex on the G6pc promoter was the target. Meanwhile, Pterosin B impaired the oxidation-reduction cycle of coenzyme Q in mitochondrial oxidative phosphorylation (OXPHOS); and antimycin A, an inhibitor of coenzyme Q: cytochrome c-oxidoreductase (termed mitochondrial complex III), also mimicked Pterosin B actions on RORα-SRC2 signaling. Although other respiratory toxins (rotenone and oligomycin) also suppressed G6pc expression accompanied by lowered ATP levels following the activation of AMP-activated kinase, minimal or no effect of these other toxins on RORα-SRC2 activity was observed.
CONCLUSIONS:
These results suggested that individual components in OXPHOS differentially linked to different transcriptional machineries for hepatic gluconeogenic programs, and the RORα-SRC2 complex acted as a sensor for oxidation-reduction cycle of coenzyme Q and regulated G6Pc expression. This was a site disrupted by Pterosin B in gluconeogenic programs. |
|
| Structure Identification: |
| J Nat Med. 2008 Jul;62(3):358-9. | | Isolation of 5-hydroxypyrrolidin-2-one and other constituents from the young fronds of Pteridium aquilinum.[Pubmed: 18437503 ] |
METHODS AND RESULTS:
5-Hydroxypyrrolidin-2-one, along with (2R)-Pterosin B, shikimic acid, kaempferol-3-O-beta-D-glucopyranoside, transtiliroside, beta-sitosterol, daucosterol, glycerol 1-stearate and benzoic acid, were isolated from the young fronds of the bracken fern Pteridium aquilinum.
CONCLUSIONS:
5-Hydroxypyrrolidin-2-one, shikimic acid and glycerol 1-stearate were isolated from the plant for the first time. | | Molecules. 2008 Feb 5;13(2):255-66. | | New benzoyl glucosides and cytotoxic pterosin sesquiterpenes from Pteris ensiformis Burm.[Pubmed: 18305416] |
METHODS AND RESULTS:
Three new compounds: 2R,3R-pterosin L 3-O-beta-D-glucopyranoside (1), beta-D-xylopyranosyl(1-->2)-7-O-benzoyl-beta-D-glucopyranoside (2) and 4-O-benzoyl-beta-D-xylo-pyranosyl(1-->2)-7-O-benzoyl-beta-D-glucopyranoside (3), together with nine known compounds, were isolated from the ethyl acetate extract of Pteris ensiformis. 5-[2-Hydroxyethylidene]-2(5H)-furanone (4), which had been synthesized, was isolated from natural sources for the first time. The structures of all isolated compounds were determined on the basis of mass and spectroscopic evidence.
CONCLUSIONS:
Compound 1 and Pterosin B (5) show cytotoxicity against HL 60 cells (human leukemia) with the IC(50) values of 3.7 and 8.7 microg/mL, respectively. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)