| In vitro: |
| Phytochem Anal. 2009 Jul-Aug;20(4):320-7. | | Antioxidant diterpenoids from the roots of Salvia barrelieri.[Pubmed: 19402189 ] | The phytochemical and biological studies carried out on Salvia species showed that their extracts and constituents have various biological activities.
The aim of this study was the isolation of diterpenoids from the roots of Salvia barrelieri Ettling and the determination of the antioxidant activity.
METHODS AND RESULTS:
Chromatographic methods were used for fractionation and isolation, respectively. Structure elucidation was established by spectroscopic methods. Five antioxidant assays were performed.
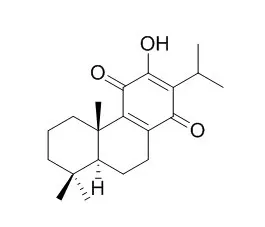
Three new abietane diterpenoids barreliol, Royleanone 12-methyl ether and 7-epi-salviviridinol, and six known diterpenoids, with a known dammarane triterpenoid, pyxinol were isolated. The absolute stereochemistry of pyxinol was confirmed by X-ray analysis.
CONCLUSIONS:
Taxodione exhibited the highest antioxidant activity among the tested compounds. | | Nat Prod Commun. 2011 May;6(5):575-9. | | Bioactivity-guided study of antiproliferative activities of Salvia extracts.[Pubmed: 21615011] | The cytotoxic activities of the n-hexane, chloroform and aqueous methanolic fractions prepared from the methanolic extract of the leaves of 23 Salvia taxa were studied for their cell growth-inhibitory activity against human cervix adenocarcinoma (HeLa), skin carcinoma (A431) and breast adenocarcinoma (MCF7) cells using the MTT assay.
METHODS AND RESULTS:
The n-hexane fractions of six Salvia taxa (S. hispanica, S. nemorosa, S. nemorosa 1. albiflora, S. pratensis, S. recognita and S. ringens) and the chloroform fraction ofS. officinalis 1. albiflora produced over 50% growth inhibition of the skin carcinoma cell line. None of the tested extracts showed substantial (above 50%) antiproliferative effects against HeLa and MCF7 cells. S. ringens was the most powerful among the studied Salvia species with a 61.8% cell growth inhibitory activity on A431 cells. In the case of S. ringens, other plant parts were also tested for antiproliferative effect, and the highest activities were recorded for the root extract. This was subjected to bioactivity-guided fractionation, which yielded four abietane diterpenes (Royleanone, horminone, 7-O-methyl-horminone and 7-acetyl-horminone), one triterpene (erythrodiol-3-acetate) and beta-sitosterol.
CONCLUSIONS:
Horminone, 7-acetyl-horminone and erythrodiol-3-acetate displayed marked concentration-dependent antiproliferative effects, while Royleanone and 7-O-methyl-horminone produced weaker activities. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)