| In vitro: |
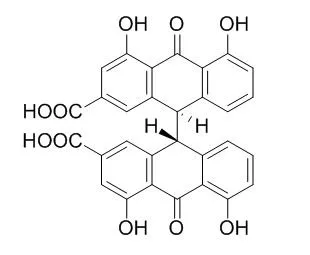
| Oncotarget. 2014 May 30;5(10):2934-46. | | Necrosis targeted combinational theragnostic approach using radioiodinated Sennidin A in rodent tumor models.[Pubmed: 24931286] | Residual cancer cells and subsequent tumor relapse is an obstacle for curative cancer treatment. Tumor necrosis therapy (TNT) has recently been developed to cause residual tumor regression or destruction.
METHODS AND RESULTS:
Here, we exploited the avidity of the Sennidin A (SA) tracer and radioiodinated SA (¹³¹I-SA) to necrotic tumors in order to further empower TNT. We showed high uptake and prolonged retention of SA in necrotic tumors and a quick clearance in other non-targeted tissues including the liver. On SPECT-CT images, tumor mass appeared persistently as a hotspot. Based on the prominent targetability of ¹³¹I-SA to the tumor necrosis, we designed a combinational theragnostic modality. The vascular disrupting agent (VDA) combretastatin A4 phosphate (CA4P) was used to cause massive tumor necrosis, which formed the target of ¹³¹I-SA that subsequently killed the residual tumor cells by cross-fire irradiation of beta particles.
Consequently, ¹³¹I-SA combined with CA4P significantly inhibited tumor growth, extended tumor doubling time and prolonged mean animal survival.
CONCLUSIONS:
In conclusion, ¹³¹I-SA in combination with necrosis inducing drugs/therapies may generate synergetic tumoricidal effects on solid malignancies by means of primary debulking and secondary cleansing process. | | Int J Mol Sci. 2015 Aug; 16(8): 18439–53. | | Identification of Hydroxyanthraquinones as Novel Inhibitors of Hepatitis C Virus NS3 Helicase[Pubmed: 26262613] | Hepatitis C virus (HCV) is an important etiological agent of severe liver diseases, including cirrhosis and hepatocellular carcinoma. The HCV genome encodes nonstructural protein 3 (NS3) helicase, which is a potential anti-HCV drug target because its enzymatic activity is essential for viral replication. Some anthracyclines are known to be NS3 helicase inhibitors and have a hydroxyanthraquinone moiety in their structures; mitoxantrone, a hydroxyanthraquinone analogue, is also known to inhibit NS3 helicase.
METHODS AND RESULTS:
Therefore, we hypothesized that the hydroxyanthraquinone moiety alone could also inhibit NS3 helicase. Here, we performed a structure–activity relationship study on a series of hydroxyanthraquinones by using a fluorescence-based helicase assay. Hydroxyanthraquinones inhibited NS3 helicase with IC50 values in the micromolar range. The inhibitory activity varied depending on the number and position of the phenolic hydroxyl groups, and among different hydroxyanthraquinones examined, 1,4,5,8-tetrahydroxyanthraquinone strongly inhibited NS3 helicase with an IC50 value of 6 μM. Furthermore, hypericin and Sennidin A, which both have two hydroxyanthraquinone-like moieties, were found to exert even stronger inhibition with IC50 values of 3 and 0.8 μM, respectively.
CONCLUSIONS:
These results indicate that the hydroxyanthraquinone moiety can inhibit NS3 helicase and suggest that several key chemical structures are important for the inhibition. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)