A new furofuran lignan, styraxlignolide B (1), and four new dibenzyl-gamma-butyrolactone lignans, styraxlignolides C, styraxlignolide D, styraxlignolide E, and Styraxlignolide F (2-5), were isolated from the EtOAc-soluble fraction of stem bark of Styrax japonica.
METHODS AND RESULTS:
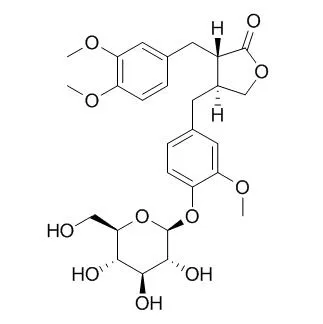
Known compounds, taraxerol (6), syringin (7), and (-)-pinoresinol glucoside (8), were also obtained. The structures of styraxligonolides B-F were determined as 2alpha-(4'-hydroxy-3'-methoxyphenyl)-6alpha-(3' ',4' '-methylenedioxyphenyl)-8-oxo-3,7-dioxabicyclo[3.3.0]octane 4'-O-(beta-D-glucopyranoside) (1), (2S,3S)-2alpha-(3' '-hydroxy-4' '-methoxybenzyl)-3beta-(4'-hydroxy-3'-methoxybenzyl)-gamma-butyrolactone 4'-O-(beta-D-glucopyranoside) (2), (2S,3S)-2alpha-(4' '-hydroxy-3' '-methoxybenzyl)-3beta-(4'-hydroxy-3'-methoxybenzyl)-gamma-butyrolactone 4'-O-(beta-D-glucopyranoside) (3), (2S,3S)-2alpha-(4' '-hydroxy-3' '-methoxybenzyl)-3beta-(4'-hydroxy-3'-methoxybenzyl)-gamma-butyrolactone 4' '-O-(beta-D-glucopyranoside) (4), and (2S,3S)-2alpha-(3' ',4' '-dimethoxybenzyl)-3beta-(4'-hydroxy-3'-methoxybenzyl)-gamma-butyrolactone 4'-O-(beta-D-glucopyranoside) (5) by spectroscopic means including 2D NMR.
CONCLUSIONS:
Compounds 1-8 were tested in vitro for antioxidant activity against 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals. Styraxlignolide C (2), styraxlignolide D (3), styraxlignolide E (4), and (-)-pinoresinol glucoside (8) exhibited weak radical-scavenging activity in the DPPH assay, with IC50 values of 380, 278, 194, and 260 microM, respectively. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)