| In vitro: |
| Molecules. 2014 May 14;19(5):6070-9. | | In vitro leishmanicidal activities of sesquiterpene lactones from Tithonia diversifolia against Leishmania braziliensis promastigotes and amastigotes.[Pubmed: 24830711 ] | Natural compounds represent a rich and promising source of novel, biologically active chemical entities for treating leishmaniasis. Sesquiterpene lactones are a recognized class of terpenoids with a wide spectrum of biological activities, including activity against Leishmania spp.
METHODS AND RESULTS:
In this work, a sesquiterpene lactone-rich preparation-a leaf rinse extract (LRE) from Tithonia diversifolia-was tested against promastigote forms of L. braziliensis. The results revealed that the LRE is a rich source of potent leishmanicidal compounds, with an LD50 value 1.5 ± 0.50 µg·mL-1. Therefore, eight sesquiterpene lactones from the LRE were initially investigated against promastigote forms of L. braziliensis. One of them did not present any significant leishmanicidal effect (LD50 > 50 µg·mL-1). Another had a cytotoxic effect against macrophages (4.5 µg·mL-1). The five leishmanicidal compounds with the highest level of selectivity were further evaluated against intracellular parasites (amastigotes) using peritoneal macrophages. Tirotundin 3-O-methyl ether, Tagitinin F, and a guaianolide reduced the internalization of parasites after 48 h, in comparison with the negative control.
CONCLUSIONS:
This is the first report on sesquiterpene lactones that have potent leishmanicidal effects on both developmental stages of L. braziliensis. | | Fitoterapia. 2012 Dec;83(8):1590-7. | | Three new sesquiterpenes from Tithonia diversifolia and their anti-hyperglycemic activity.[Pubmed: 22986291 ] |
METHODS AND RESULTS:
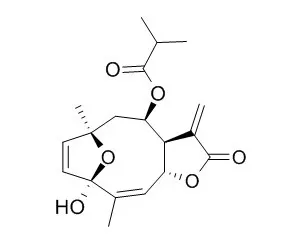
Three new germacrane sesquiterpenes (1), (2), (3), along with eleven known sesquiterpenes, namely, tirotundin-3-O-methyl ether (4), deacetylvguiestin (5), 1β-hydroxydiversifolin-3-O-methyl ether (6), tagitinin C (7), 1β-hydroxytirotundin-3-O-methyl ether (8), 1β-hydroxytirotundin-1,3-O-dimethyl ether (9), Tagitinin F-3-O-methyl ether (10), Tagitinin F (11), tagitinin A (12), 3β-acetoxy-4α-hydroxyeduesm-11(13)-en-12-oic acid (13) and ilicic acid (14) were isolated from the aerial parts of Tithonia diversifolia. Their structures were established by spectroscopic analysis, while the relative configuration of compound 1 was confirmed by X-ray diffraction analysis. In addition, compounds 1-14 were evaluated in vitro for their anti-hyperglycemic activity by glucose uptake in 3T3-L1 adipocytes.
CONCLUSIONS:
It was found that 10μg/mL 1, 3, 6 and 8 could significantly increase glucose uptake without significant toxic effects. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)