| Description: |
Trans-caffeic acid stearyl ester is posited to inhibit melanogenesis signaling while suppressing cAMP levels and, subsequently, MC1R, MITF, tyrosinase, TRP-2 and TRP-1 down-regulation, resulting in the suppression of tyrosinase activity, DOPA oxidase activity and melanin synthesis. |
| In vitro: |
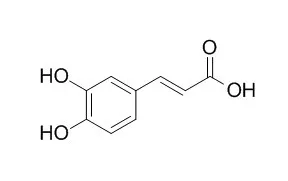
| Biol Pharm Bull. 2012;35(12):2198-203. | | trans-Caffeic acid stearyl ester from Paeonia suffruticosa inhibits melanin synthesis by cAMP-mediating down-regulation of α-melanocyte-stimulating hormone-stimulated melanogenesis signaling pathway in B16 cells.[Pubmed: 23207771] | Trans-caffeic acid stearyl ester (TCASE) from the root cortex of Paeonia suffruticosa ANDREWS is a traditional medicinal herb that has several beneficial properties. However, the inhibitory effect of Trans-caffeic acid stearyl ester on melanogenesis has not been explored.
METHODS AND RESULTS:
In the cell viability assay, Trans-caffeic acid stearyl ester did not show a cytotoxic effect at a dose of 65 µM for 48 h in B16, HaCaT and Hs68 cells. Trans-caffeic acid stearyl ester considerably inhibits melanin synthesis, and reduces intracellular cyclic adenosine monophosphate (cAMP) levels, tyrosinase activity and L-3-(3,4-dihydroxyphenyl)-alanine (DOPA) oxidase activity in a concentration-dependent manner in the presence of α-melanocyte-stimulating hormone (α-MSH) in B16 cells, and the inhibition efficiency of Trans-caffeic acid stearyl ester exceeds that of ascorbic acid and arbutin. Trans-caffeic acid stearyl ester reduces melanocortin-1 receptor (MC1R), microphthalmia transcription factor (MITF), tyrosinase, tyrosinase-related protein-2 (TRP-2) and TRP-1 mRNA and protein levels in B16 cells.
CONCLUSIONS:
Based on the findings, Trans-caffeic acid stearyl ester is posited to inhibit melanogenesis signaling while suppressing cAMP levels and, subsequently, MC1R, MITF, tyrosinase, TRP-2 and TRP-1 down-regulation, resulting in the suppression of tyrosinase activity, DOPA oxidase activity and melanin synthesis. | | Applied Catalysis B Environmental , 2016 , 182 :347-355. | | Photochemical and photocatalytic isomerization of trans-caffeic acid and cyclization of cis-caffeic acid to esculetin[Reference: WebLink] |
METHODS AND RESULTS:
The photoisomerization of Trans-caffeic acid to cis-caffeic acid has been studied in the presence of N2 in homogeneous aqueous solutions and in suspensions of various TiO2 catalysts. The results supported the hypothesis of an energy transfer process from TiO2 to the substrate due to the recombination of the photogenerated electron–hole pairs. The differences among the measured photostationary [cis]/[trans] ratios have been attributed to the different physico-chemical properties of the catalysts. In particular, the lowest ratio measured in the presence of Merck TiO2 was ascribed to the very low adsorption of Trans-caffeic acid onto the surface of this sample.
CONCLUSIONS:
In the presence of O2 and at alkaline pHs, cis-caffeic acid cyclized to esculetin both in the absence and in the presence of irradiation. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)