| In vivo: |
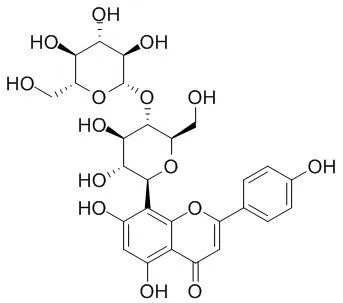
| J Chromatogr B Analyt Technol Biomed Life Sci. 2010 Jul 1;878(21):1837-44. | | Simultaneous determination of vitexin-4''-O-glucoside, vitexin-2''-O-rhamnoside, rutin and vitexin from hawthorn leaves flavonoids in rat plasma by UPLC-ESI-MS/MS.[Pubmed: 20570577] | A sensitive and accurate ultra-performance liquid chromatography electrospray ionization tandem mass spectrometry (UPLC-ESI-MS/MS) method was developed and validated for the simultaneous determination of Vitexin -4''-O-glucoside (VGL), vitexin-2''-O-rhamnoside (VRH), rutin (RUT) and vitexin (VIT) in rat plasma after intravenous administration of hawthorn leaves flavonoids (HLF).
METHODS AND RESULTS:
Following protein precipitation by methanol, the analytes were separated on an ACQUITY UPLC BEH C(18) column packed with 1.7 microm particles by gradient elution using a mobile phase composed of acetonitrile and water (containing 0.1% formic acid) at a flow rate of 0.20 mL/min. The analytes and diphenhydramine (internal standard, IS) were detected in the multiple reaction monitoring (MRM) mode by means of an electrospray ionization (ESI) interface (m/z 292.96 for Vitexin -4''-O-glucoside , m/z 293.10 for vitexin-2''-O-rhamnoside, m/z 299.92 for rutin, m/z 310.94 for vitexin and m/z 166.96 for IS). The calibration curve was linear over the range 10-40,000 ng/mL for vitexin-4''-O-glucoside, 10-50,000 ng/mL for vitexin-2''-O-rhamnoside, 8-1000 ng/mL for rutin and 16-2000 ng/mL for vitexin. The intra- and inter-run precisions (relative standard deviation, RSD) of these analytes were all within 15% and the accuracy (the relative error, RE) ranged from -10% to 10%.
CONCLUSIONS:
The stability experiment indicated that the four analytes in rat plasma samples and plasma extracts under anticipated conditions were stable. The developed method was applied for the first time to pharmacokinetic studies of the four bioactive compounds of hawthorn leaves flavonoids following a single intravenous administration of 20 mg/kg in rats. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)