| Structure Identification: |
| Journal of bacteriology,1987,169(11):4972-9. | | Bacterial metabolism of alpha-pinene: pathway from alpha-pinene oxide to acyclic metabolites in Nocardia sp. strain P18.3.[Reference: WebLink] |
METHODS AND RESULTS:
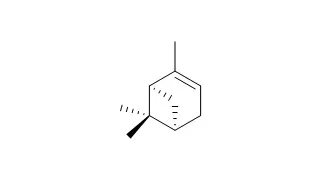
Over 20 gram-positive bacteria were isolated by elective culture with (+/-)-alpha-pinene((+)-alpha-Pinene/(-)-alpha-Pinene) as the sole carbon source. One of these strains, Nocardia sp. strain P18.3, was selected for detailed study. alpha-Pinene-grown cells oxidized, without lag, alpha-pinene, alpha-pinene oxide (epoxide), and the cis and trans isomers of 2-methyl-5-isopropylhexa-2,5-dienal. No other tested terpene was oxidized at a significant rate. alpha-Pinene was not metabolized by cell extracts in the presence or absence of NADH or NADPH. Cell extracts catalyzed a rapid decyclization of alpha-pinene oxide, in the absence of added cofactors, with the formation of cis-2-methyl-5-isopropylhexa-2,5-dienal. Further oxidation of the aldehyde to the corresponding acid occurred in the presence of NAD. Both activities were induced by growth with alpha-pinene.
CONCLUSIONS:
A rapid, nonenzymic transformation of the cis aldehyde into the trans isomer occurred in glycine buffer. The trans isomer was also a substrate for the NAD-linked aldehyde dehydrogenase.
The distribution of the alpha-pinene oxide lyase in alpha-pinene-utilizing Pseudomonas spp. was also investigated and was compatible with the two alternative ring-cleavage sequences that have been proposed on the basis of accumulated metabolites. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)