| In vitro: |
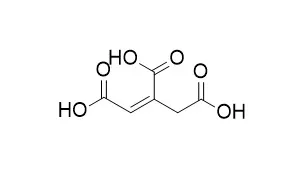
| Applied Microbiology and Biotechnology, 2008, 80(2):223-229. | | Cloning and functional characterization of the cis-aconitic acid decarboxylase (CAD) gene from Aspergillus terreus.[Reference: WebLink] | A filamentous fungus Aspergillus terreus produces itaconic acid, which is predicted to be derived from cis-Aconitic acid via catalysis by cis-Aconitic acid decarboxylase (CAD) in the carbon metabolism of the fungus.
METHODS AND RESULTS:
To clarify the enzyme's function and a pathway for itaconic acid biosynthesis, we cloned a novel gene encoding the enzyme. The open reading frame of this gene (CAD1) consists of 1,529 bp encoding 490 amino acids and is interrupted by a single intron. Among the identified proteins in the database, the primary structure of the protein encoded by CAD1 shared high identity with the MmgE/PrpD family of proteins, including a number of 2-methylcitrate dehydratases of bacteria. The cloned gene excluding an intron was introduced into the expression plasmid pAUR-CAD1 controlled by the ADH1 promoter. The CAD activity in Saccharomyces cerevisiae was confirmed by directly detecting itaconic acid as a product from cis-Aconitic acid as a substrate.
CONCLUSIONS:
This result reveals for the first time that this gene encodes CAD, which is essential for itaconic acid production in A. terreus. | | AIDS Research and Human Retroviruses, 01 Jun 1996, 12(9):769-775. | | Antiviral effects of milk proteins: acylation results in polyanionic compounds with potent activity against human immunodeficiency virus types 1 and 2 in vitro.[Reference: WebLink] |
METHODS AND RESULTS:
A number of native and modified milk proteins from bovine or human sources were analyzed for their inhibitory effects on human immunodeficiency virus type 1 (HIV-1) and HIV-2 in vitro in an MT4 cell test system. The proteins investigated were lactoferrin, alpha-lactalbumin, beta-lactoglobulin A, and beta-lactoglobulin B. By acylation of the amino function of the lysine residues in the proteins, using anhydrides of succinic acid or cis-Aconitic acid, protein derivatives were obtained that all showed a strong antiviral activity against human immunodeficiency virus type 1 and/or 2. The in vitro IC50 values of the aconitylated proteins were in the concentration range of 0.3 to 3 nM. Succinylation or aconitylation of alpha-lactalbumin and beta-lactoglobulin A/B also produced strong anti-HIV-2 activity with IC50 values on the order 500 to 3000 nM. All compounds showed virtually no cytotoxicity at the concentration used. Peptide-scanning studies indicated that the native lactoferrin as well as the charged modified proteins strongly bind to the V3 loop of the gp120 envelope protein, with Kd values in the same concentration range as the above-mentioned IC50.
CONCLUSIONS:
Therefore, shielding of this domain, resulting in inhibition of virus-cell fusion and entry of the virus into MT4 cells, may be the likely underlying mechanism of antiviral action. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)