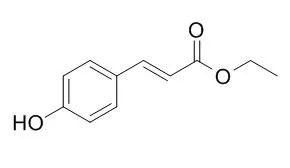
| Structure Identification: |
| J Agric Food Chem. 2015 Apr 8;63(13):3402-18. | | Sensomics analysis of key bitter compounds in the hard resin of hops (Humulus lupulus L.) and their contribution to the bitter profile of Pilsner-type beer.[Pubmed: 25793563] | Recent brewing trials indicated the occurrence of valuable bitter compounds in the hard resin fraction of hop.
METHODS AND RESULTS:
Aiming at the discovery of these compounds, hop's ε-resin was separated by means of a sensory guided fractionation approach and the key taste molecules were identified by means of UV/vis, LC-TOF-MS, and 1D/2D-NMR studies as well as synthetic experiments. Besides a series of literature known xanthohumol derivatives, multifidol glucosides, flavon-3-on glycosides, and p-coumaric acid esters, a total of 11 bitter tastants are reported for the first time, namely, 1",2"-dihydroxanthohumol F, 4'-hydroxytunicatachalcone, isoxantholupon, 1-methoxy-4-prenylphloroglucinol, dihydrocyclohumulohydrochinone, xanthohumols M, N, and P, and isoxanthohumols M, N, and P, respectively. Human sensory analysis revealed low bitter recognition threshold concentrations ranging from 5 (co-multifidol glucopyranoside) to 198 μmol/L (trans-p-Coumaric acid ethyl ester) depending on their chemical structure. For the first time, LC-MS/MS quantitation of these taste compounds in Pilsner-type beer, followed by taste re-engineering experiments, revealed the additive contribution of iso-α-acids and the identified hard resin components to be truly necessary and sufficient for constructing the authentic bitter percept of beer.
CONCLUSIONS:
Finally, brewing trails using the ε-resin as the only hop source impressively demonstrated the possibility to produce beverages strongly enriched with prenylated hop flavonoids. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)