| Structure Identification: |
| J Nat Prod. 1981 May-Jun;44(3):312-9. | | 19-Hydroxybaccatin III, 10-deacetylcephalomannine, and 10-deacetyltaxol: new antitumor taxanes from Taxus wallichiana.[Pubmed: 7264680] |
METHODS AND RESULTS:
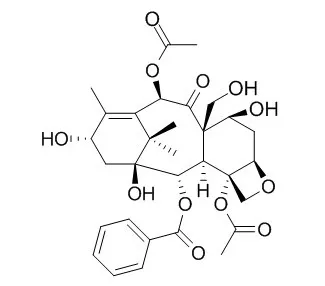
Activity-guided, chromatographic fractionation for a polar extract of Taxus wallichiana Zucc. (originally identified as Cephalotaxus mannii Hook.) resulted in the isolation of three new KB cytotoxic taxane derivatives. Nmr and ms spectral analyses permitted their characterization as 19-Hydroxybaccatin III (3), 10-deacetylcephalomannine (4), and 10-deacetyltaxol (5).
CONCLUSIONS:
The latter two compounds, which are also active against PS leukemia in vivo, were observed to be especially labile, each forming equilibrium mixtures with their cytotoxic C-7 epimers (9, 10). | | Chem Pharm Bull (Tokyo). 1995 Feb;43(2):365-7. | | Taxol and its related taxoids from the needles of Taxus sumatrana.[Pubmed: 7728941] |
METHODS AND RESULTS:
Through bioassay-guided separation of the chemical constituents of the needles of Taxus sumatrana, taxol (1), cephalomannine (2), and a new taxoid 19-hydroxy-13-oxobaccatin III (8) have been isolated together with 7-epi-10-deacetyltaxol (3), 7-epi-10-deacetylcephalomannine (4), baccatin III (5), 19-Hydroxybaccatin III (6), and 10-deacetyl-13-oxobaccatin III (7). The chemical structure of 8 has been elucidated on the bases of its chemical and physicochemical properties. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)