| Description: |
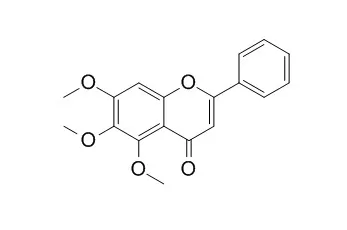
5,6,7-Trimethoxyflavone exhibits relatively high inhibitory effects on herpes simplex virus type 1 (HSV-1), human cytomegalovirus and poliovirus, it and acylovir were synergic in their anti-HSV activities at levels below the 50% inhibitory concentrations for antiviral activity.
5,6,7-Trimethoxyflavone down-regulates the expressions of the pro-inflammatory iNOS, COX-2, TNF-α, IL-1β, and IL-6 genes in macrophages by interfering with the activation of NF-κB, AP-1, and STAT1/3.
|
| In vitro: |
| Journal of Antimicrobial Chemotherapy,1997,39(6):821–824. | | Antiviral activity of 5,6,7-trimethoxyflavone and its potentiation of the antiherpes activity of acyclovir.[Reference: WebLink] |
METHODS AND RESULTS:
A naturally occurring flavone, 5,6,7-Trimethoxyflavone (TMF), isolated from the plant Callicarpa japonica, was subjected to antiviral assays. The compound exhibited relatively high inhibitory effects on herpes simplex virus type 1 (HSV-1), human cytomegalovirus and poliovirus. The anti-HSV-1 action was not due to the inhibition of virus adsorption, entry and viral protein synthesis, but might involve, at least in part, a virucidal activity, which results in a suppression of viral binding to host cells at an early replication stage.
CONCLUSIONS:
TMF and acylovir were synergic in their anti-HSV activities at levels below the 50% inhibitory concentrations for antiviral activity. | | Food & Chemical Toxicology, 2013, 62:847-855. | | 5,6,7-trimethoxyflavone suppresses pro-inflammatory mediators in lipopolysaccharide-induced RAW 264.7 macrophages and protects mice from lethal endotoxin shock.[Reference: WebLink] | 5,6,7-Trimethoxyflavone (TMF), methylations of the hydroxyl groups of oroxylin A or baicalein, was found to significantly inhibit the productions of nitric oxide (NO) and prostaglandin E2 (PGE2) in lipopolysaccharide (LPS)-treated RAW 264.7 macrophages. However, no report has been issued on the anti-inflammatory potential of TMF and the underlying molecular mechanism.
METHODS AND RESULTS:
In the present study, we investigated the anti-inflammatory effects of TMF in LPS-induced RAW 264.7 macrophages and LPS-induced septic shock in mice. TMF dose-dependently inhibits iNOS and COX-2 at the protein, mRNA, and promoter binding levels and that these inhibitions cause attendant decreases in the productions of NO and PGE2. TMF inhibits the productions and mRNA expressions of tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 induced by LPS. Furthermore, TMF suppress the transcriptional activity of nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1), and nuclear translocations of NF-κB, AP-1, and signal transducer and activator of transcription 1/3 (STAT1/3). Pretreatment with TMF increase the survival rate of mice with LPS-induced endotoxemia and reduced the serum levels of cytokines.
CONCLUSIONS:
Taken together, these findings suggest that TMF down-regulates the expressions of the pro-inflammatory iNOS, COX-2, TNF-α, IL-1β, and IL-6 genes in macrophages by interfering with the activation of NF-κB, AP-1, and STAT1/3. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)