| Structure Identification: |
| Bioorganic & medicinal chemistry letters, 2012, 22(5):2125-2129. | | Small molecule inhibitors of the HPV16-E6 interaction with caspase 8.[Reference: WebLink] |
METHODS AND RESULTS:
High-risk strains of human papillomaviruses (HPVs) cause nearly all cases of cervical cancer as well as a growing number of head and neck cancers. The oncogenicity of these viruses can be attributed to the activities of their two primary oncoproteins, E6 and E7. The E6 protein has among its functions the ability to prevent apoptosis of infected cells through its binding to FADD and caspase 8. A small molecule library was screened for candidates that could inhibit E6 binding to FADD and caspase 8. Flavonols were found to possess this activity with the rank order of myricetin > morin > quercetin > kaempferol = galangin ≫ (apigenin, 7-Hydroxyflavonol, rhamnetin, isorhamnetin, geraldol, datiscetin, fisetin, 6-hydroxyflavonol). Counter screening, where the ability of these chosen flavonols to inhibit caspase 8 binding to itself was assessed, demonstrated that myricetin, morin and quercetin inhibited GST-E6 and His-caspase 8 binding in a specific manner.
CONCLUSIONS:
The structure–activity relationships suggested by these data are unique and do not match prior reports on flavonols in the literature for a variety of anticancer assays. |
|


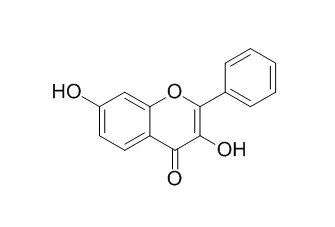



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)