| Structure Identification: |
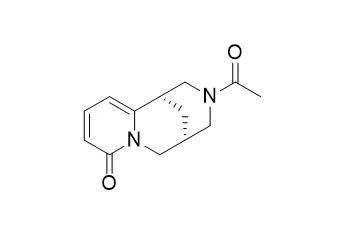
| J Chem Ecol . 1993 Mar;19(3):441-448. | | Quinolizidine alkaloids inGenista acanthoclada and its holoparasite,Cuscuta palaestina[Pubmed: 24248948] | | About 20 quinolizidine alkaloids were identified inGenista acanthoclada by capillary GLC and GLC-MS, such as sparteine, 11,12-dehy-drosparteine, retamine,N-methylcytisine, cytisine, 17-oxosparteine, lupanine,α-isolupanine, 5,6-dehydrolupanine, 10-oxosparteine,N-carbomethoxycytisine, 17-oxoretamine,N-formylcytisine,N-Acetylcytisine, and anagyrine. Its phloem-feeding holoparasiteCuscuta palaestina contained alkaloids too, such as sparteine, 11,12-dehydrosparteine, retamine,N-methylcytisine, cytisine, 17-oxosparteine, lupanine,N-carbomethoxycytisine, and anagyrine. Whereas sparteine, retamine, 17-oxosparteine, and cytisine are the main alkaloids ofG. acanthoclada, lupanine, cytisine,N-methylcytisine, and anagyrine are abundant and enriched inC. palaestina. Since these alkaloids figure as antiherbivoral chemical defense compounds inGenista, it is assumed that the parasite can exploit the acquired allelochemicals for its own protection. | | Rapid Commun Mass Spectrom . 2007;21(8):1409-1413. | | Electron ionization mass spectral study of selected N-amide and N-alkyl derivatives of cytisine[Pubmed: 17370243] | | (-)-Cytisine and its derivatives are promising alkaloids in the development of new drugs for the treatment of disorders of the central nervous system (CNS). Electron ionization (EI) mass spectral fragmentations of cytisine (1), N-methylcytisine (2), N-ethylcytisine (3), N-Acetylcytisine (4), N-propionylcytisine (5) and N-benzoylcytisine (6) have been investigated. Detailed fragmentation pathways have been identified for all significant ions, including a few characteristic fragment ions. The principal fragmentation routes of compounds 1-6 have been determined on the basis of EI low-resolution, high-resolution and B2/E linked scans as well as linked scans at constant B/E. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)