| In vitro: |
| Biochem Biophys Res Commun. 2015 Jan 9;456(2):621-5. | | Betaine is a positive regulator of mitochondrial respiration.[Pubmed: 25498545] | Betaine protects cells from environmental stress and serves as a methyl donor in several biochemical pathways. It reduces cardiovascular disease risk and protects liver cells from alcoholic liver damage and nonalcoholic steatohepatitis. Its pretreatment can rescue cells exposed to toxins such as rotenone, chloroform, and LiCl. Furthermore, it has been suggested that Betaine can suppress cancer cell growth in vivo and in vitro. Mitochondrial electron transport chain (ETC) complexes generate the mitochondrial membrane potential, which is essential to produce cellular energy, ATP. Reduced mitochondrial respiration and energy status have been found in many human pathological conditions including aging, cancer, and neurodegenerative disease.
METHODS AND RESULTS:
In this study we investigated whether Betaine directly targets mitochondria. We show that Betaine treatment leads to an upregulation of mitochondrial respiration and cytochrome c oxidase activity in H2.35 cells, the proposed rate limiting enzyme of ETC in vivo. Following treatment, the mitochondrial membrane potential was increased and cellular energy levels were elevated.
CONCLUSIONS:
We propose that the anti-proliferative effects of Betaine on cancer cells might be due to enhanced mitochondrial function contributing to a reversal of the Warburg effect.
| | Int J Oncol. 2014 Sep;45(3):1250-6. | | Anti-inflammatory effects of betaine on AOM/DSS‑induced colon tumorigenesis in ICR male mice.[Pubmed: 24969167] | Betaine is an important human nutrient obtained from various foods and studies in animals and humans have provided results suggesting their pathogenesis of various chronic diseases and points to a role in risk assessment and disease prevention. However, the molecular mechanisms of its activity remain poorly understood and warrant further investigation.
METHODS AND RESULTS:
This study was performed to investigate the anti-inflammation and tumor preventing capacity of Betaine on colitis-associated cancer in mice. In in vivo experiments, we induced colon tumors in mice by azoxymethane (AOM) and dextran sulfate sodium (DSS) and evaluated the effects of Betaine on tumor growth. Administration with Betaine significantly decreased the incidence of tumor formation with downregulation of inflammation. Treatment with Betaine inhibited ROS generation and GSSG concentration in colonic mucosa. Based on the qPCR data, administration of Betaine inhibited inflammatory cytokines such TNF-α, IL-6, iNOS and COX-2. In in vitro experiments, LPS-induced NF-κB and inflammatory-related cytokines were inhibited by Betaine treatment in RAW 264.7 murine macrophage cells.
CONCLUSIONS:
Our findings suggest that Betaine is one of the candidates for the prevention of inflammation-associated colon carcinogenesis. |
|
| In vivo: |
| Br J Pharmacol. 2014 Sep;171(17):4073-86. | | Rectification of impaired adipose tissue methylation status and lipolytic response contributes to hepatoprotective effect of betaine in a mouse model of alcoholic liver disease.[Pubmed: 24819676] | Overactive lipolysis in adipose tissue contributes to the pathogenesis of alcoholic liver disease (ALD); however, the mechanisms involved have not been elucidated. We previously reported that chronic alcohol consumption produces a hypomethylation state in adipose tissue. In this study we investigated the role of hypomethylation in adipose tissue in alcohol-induced lipolysis and whether its correction contributes to the well-established hepatoprotective effect of Betaine in ALD.
METHODS AND RESULTS:
Male C57BL/6 mice were divided into four groups and started on one of four treatments for 5 weeks: isocaloric pair-fed (PF), alcohol-fed (AF), PF supplemented with Betaine (BT/AF) and AF supplemented with Betaine (BT/AF). Betaine, 0.5% (w v(-1) ), was added to the liquid diet. Both primary adipocytes and mature 3T3-L1 adipocytes were exposed to demethylation reagents and their lipolytic responses determined.
Betaine alleviated alcohol-induced pathological changes in the liver and rectified the impaired methylation status in adipose tissue, concomitant with attenuating lipolysis. In adipocytes, inducing hypomethylation activated lipolysis through a mechanism involving suppression of protein phosphatase 2A (PP2A), due to hypomethylation of its catalytic subunit, leading to increased activation of hormone-sensitive lipase (HSL). In line with in vitro observations, reduced PP2A catalytic subunit methylation and activity, and enhanced HSL activation, were observed in adipose tissue of alcohol-fed mice. Betaine attenuated this alcohol-induced PP2A suppression and HSL activation.
CONCLUSIONS:
In adipose tissue, a hypomethylation state contributes to its alcohol-induced dysfunction and an improvement in its function may contribute to the hepatoprotective effects of Betaine in ALD. | | Anticancer Res. 2015 Mar;35(3):1475-80. | | Sulfobetaine (dimethylsulfoniopropionate) and glycine betaine show incompatible involvement in crucial Ehrlich ascites carcinoma in mice.[Pubmed: 25750300] |
METHODS AND RESULTS:
The role of methylation reactions in cancer was examined using the methylating agents, sulfoBetaine [dimethylsulfonioproponate (DMSP)], and glycine Betaine (GB), in murine crucial Ehrlich ascites carcinoma (EAC) for up to 10 days.
DMSP administration in EAC-bearing mice mitigated EAC, while GB administration clearly promoted EAC. However, the immune cell profiles did not differ largely between animals receiving DMSP and those receiving GB. Moreover, DMSP and GB had merely any effects on proliferation of EAC cells in vitro. Injection of DMSP into normal mice interestingly led to macrophage accumulation in the peritoneal cavity in a dose-dependent manner at early rearing.
CONCLUSIONS:
These results indicate that GB promoted EAC by the methylation of cancer promotor gene, whereas DMSP ameliorated EAC by the accumulation of activated macrophages with a rapid response and long life span during cancer progression. | | Gastroenterology. 2003 May;124(5):1488-99. | | Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice.[Pubmed: 12730887] | Alcohol-induced hyperhomocysteinemia has been reported in rats and humans. Hyperhomocysteinemia has been associated with endoplasmic reticulum (ER) stress leading to the activation of ER-dependent apoptosis or up-regulation of lipid synthesis. This novel ER stress mechanism of alcoholic liver injury was studied in the model of intragastric alcohol-fed mice.
METHODS AND RESULTS:
Effects of alcohol on gene expression were analyzed using cDNA microarrays, RT-PCR, and Western blots over a period of 6 weeks. Liver injury was examined by histologic staining and TUNEL.
We observed fatty liver, increased hepatic necroinflammation and apoptosis, and hyperhomocysteinemia. Of 1176 toxicology-related genes, glucose-regulated proteins (GRP-78 and -94), growth arrest/DNA damage-inducible protein 153 (CHOP/GADD153), and caspase-12 indicative of an ER stress response were among the alcohol-responsive genes. Sterol regulatory element binding protein (SREBP-1) and HMG-CoA reductase also were enhanced with alcohol administration. RT-PCR and selective Western blots confirmed the alcohol-induced expression of ER stress-related apoptosis and lipid synthesis genes. Addition of 0.5% and maximal 1.5% Betaine to the alcohol diet reduced the elevated level of plasma homocysteine by 54% and more than 80% accompanied by a decrease in hepatic lipids and ER stress response. Betaine did not attenuate the ethanol-induced increase in tumor necrosis factor alpha or CD14 mRNA.
CONCLUSIONS:
The results strongly suggest that alcohol may modulate both apoptotic and fat synthetic gene expression through homocysteine-induced ER stress in chronic alcoholic mouse liver and that correction of hyperhomocysteinemia by Betaine or other approaches may be useful to prevent alcoholic liver disease. |
|


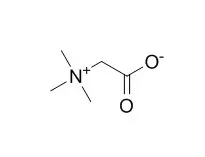



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)