| In vitro: |
| J Nat Prod. 1996 Sep;59(9):834-8. | | Novel antiplatelet constituents from formosan moraceous plants.[Pubmed: 8864236] | Sixteen constituents from Formosan Moraceous plants were tested for their antiplatelet activities in rabbit platelet suspension and human platelet-rich plasma.
METHODS AND RESULTS:
Cycloartocarpin A, cycloheterophyllin, broussochalcone A, kazinol A, broussoaurone A, and Broussoflavonol F showed strong inhibition of arachidonic acid (AA)-induced platelet aggregation.
CONCLUSIONS:
Of the compounds tested, broussochalcone A exhibited the most potent inhibition of platelet aggregation induced by AA (IC50 = 6.8 microM). The antiplatelet effects of cycloheterophyllin, broussochalcone A, kazinol B, broussoaurone A, and Broussoflavonol F are partially due to an inhibitory effect on cyclooxygenase. | | Journal of Chemical and Pharmaceutical Research, 2014, 6(6):688-69. | | Antioxidant activity of flavonoid compounds from the leaves of Macaranga gigantea[Reference: WebLink] |
METHODS AND RESULTS:
Three flavonoid compounds have been isolated from the leaves of Macaranga gigantea (Euphorbiaceae) namely as glyasperin A (1), Broussoflavonol F (2), apigenin (3). Their structures were elucidated by spectroscopic methods including UV, IR, HRESIMS, 1D and 2D NMR analysis. Compounds 1–3 were evaluated for their radical scavenging against 2,2-diphenyl-1-picrylhydrazyl (DPPH), showing their IC50 were 125.10, 708.54, and 518.01 μM, respectively.
CONCLUSIONS:
The results indicate that as glyasperin A (1) more active than ascorbic acid (329.01 μM). | | Food Chemistry, 2008 , 106 (2) :529-535. | | Tyrosinase inhibitors from paper mulberry (Broussonetia papyrifera)[Reference: WebLink] |
METHODS AND RESULTS:
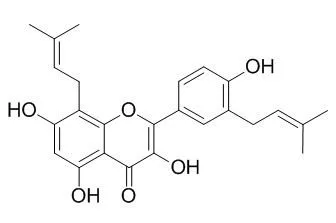
Fractionation of a chloroform-soluble extract from twigs of Broussonetia papyrifera, led to the isolation of one new compound, 3,5,7,4'-tetrahydroxy-3'-(2-hydroxy-3-methylbut-3-enyl)flavone (1), and 10 known compounds, uralenol (2), quercetin (3), isolicoflavonol (4), papyriflavonol A (5), Broussoflavonol F (6), 5,7,3',5'-tetrahydroxyflavanone (7), luteolin (8), isoliquiritigenin (9), broussochalcone A (10) and 5,7,3',4'-tetrahydroxy-3-methoxyflavone (11). Their structures were identified by interpretation of MS, 1H NMR, 13C NMR, HMQC and HMBC data.
CONCLUSIONS:
Their inhibitory activities on mushroom tyrosinase using l-tyrosine as substrate were investigated and the IC₅₀ values of 3,5,7,4'-tetrahydroxy-3'-(2-hydroxy-3-methylbut-3-enyl)flavone, uralenol, quercetin and Broussoflavonol F were found to be 96.6, 49.5, 57.8, and 82.3 μM, respectively, better than arbutin, a well-known tyrosinase inhibitor. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)