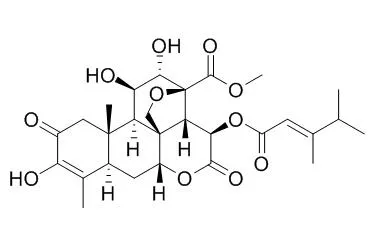
| Description: |
Bruceantin has antiviral activity, it can inhibit pepper mottle virus in pepper; it also shows high antimalarial activity. Bruceantin is an effective agent in controlling the proliferation, viability and migration of multiple myeloma cancer stem cells (CSCs) as well as angiogenesis in vitro. Bruceantin exhibits NF-κB p65 inhibition, and cytotoxic potential against HT-29, HeLa, and HL-60 cells . |
| In vitro: |
| Virus Res. 2017 Jan 2;227:49-56. | | Quassinoids isolated from Brucea javanica inhibit pepper mottle virus in pepper.[Pubmed: 27686478 ] |
METHODS AND RESULTS:
A green fluorescent protein (GFP)-tagged pepper mottle virus (PepMoV) based leaf-disc method and systemic host method were developed to identify antiviral agents. Preliminary experiments using a PepMoV-GFP based leaf-disc method led to the isolation of five quassinoids, including brusatol (1), Bruceantin (2), brucein A (3), Bruceantinol (4), and brucein B (5), from the CH3OH extract of Brucea javanica.
CONCLUSIONS:
All isolated compounds exhibited inactivation effects in systemic host plants, and compounds 3 and 4 were potent, with a minimum inhibitory concentration of 10μM. Furthermore, compound 3 was found to have a protective effect at the tested concentration of 40μM. | | Cancer Biol Ther. 2016 Sep;17(9):966-75. | | Bruceantin inhibits multiple myeloma cancer stem cell proliferation.[Pubmed: 27434731 ] |
Multiple myeloma (MM) continues to claim the lives of a majority of patients. MM cancer stem cells (CSCs) have been demonstrated to sustain tumor growth. Due to their ability to self-renew and to express detoxifying enzymes and efflux transporters, MM-CSCs are rendered highly resistant to conventional therapies. Therefore, managing MM-CSCs characteristics could have profound clinical implications. Bruceantin (BCT) is a natural product previously demonstrated to inhibit the growth of MM in RPMI 8226 cells-inoculated mouse xenograft models, and to cause regression in already established tumors.
METHODS AND RESULTS:
The objectives of the present study were to test the inhibitory effects of BCT on MM-CSCs growth derived from a human primary tumor, and to explore a mechanism of action underlying these effects. BCT exhibited potent antiproliferative activity in MM-CSCs starting at 25 nM. BCT induced cell cycle arrest, cell death and apoptosis in MM-CSCs as well as inhibited cell migration and angiogenesis in vitro. Using a qPCR screen, it was found that the gene expression of a number of Notch pathway members was altered. Pretreatment of MM-CSCs with the γ-secretase inhibitor RO4929097, a Notch pathway inhibitor, reversed BCT-induced effects on MM-CSCs proliferation.
CONCLUSIONS:
In this study, BCT was shown to be an effective agent in controlling the proliferation, viability and migration of MM-CSCs as well as angiogenesis in vitro. The effect on MM-CSCs proliferation may be mediated by the Notch pathway. These results warrant further investigation of BCT in a broader set of human-derived MM-CSCs and with in vivo models representative of MM. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)