| Description: |
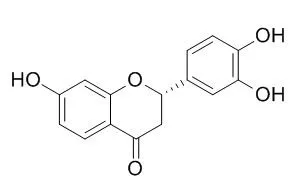
Butin has antioxidant activity, can protect cells against H2O2-induced apoptosis, oxidative DNA damage and oxidative mitochondrial dysfunction; it attenuates oxidative stress by activating Nrf2-mediated Mn SOD induction via the PI3K/Akt signaling pathway.Butin against H2O2-induced apoptosis were exerted via blockade of membrane potential depolarization, inhibition of the JNK pathway and mitochondria-involved caspase-dependent apoptotic pathway, enhancing the expression of phosphorylated Akt (active form of Akt), a regulator of OGG1.
|
| In vitro: |
| J Cell Biochem. 2012 Jun;113(6):1987-97. | | The cytoprotective effect of butin against oxidative stress is mediated by the up-regulation of manganese superoxide dismutase expression through a PI3K/Akt/Nrf2-dependent pathway.[Pubmed: 22253095] | Butin (7,3',4'-trihydroxydihydroflavone), a flavonoid with antioxidant activity, was recently reported to protect cells against H2O2-induced apoptosis, oxidative DNA damage and oxidative mitochondrial dysfunction. The objective of the present study was to elucidate the mechanism by which Butin protects mitochondria.
METHODS AND RESULTS:
The antioxidant function of manganese superoxide dismutase (Mn SOD) is important in preventing oxidative stress. While exposure to H2O2 reduced the expression of Mn SOD in Chinese hamster lung fibroblast (V79-4), the addition of Butin restored Mn SOD expression at both the mRNA and protein levels, resulting in increased Mn SOD activity. The transcription factor NF-E2-related factor 2 (Nrf2) regulates Mn SOD gene expression by binding to the antioxidant responsive element (ARE). Butin enhanced the nuclear translocation and ARE-binding activity of Nrf2, which was decreased by H2O2. The siRNA-mediated knockdown of Nrf2 attenuated Butin-induced Mn SOD expression and activity. Further, phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB, Akt) contributed to the ARE-driven Mn SOD expression. Butin activated PI3K/Akt and exposure to either LY294002 (a PI3K inhibitor), Akt inhibitor IV (an Akt-specific inhibitor), or Akt siRNA suppressed the Butin-induced activation of Nrf2, resulting in decreased Mn SOD expression and activity. Finally, the cytoprotective effect of Butin against H2O2-induced cell damage was suppressed by the siRNA-mediated knockdown of Mn SOD.
CONCLUSIONS:
These studies demonstrate that Butin attenuates oxidative stress by activating Nrf2-mediated Mn SOD induction via the PI3K/Akt signaling pathway. | | Food Chem Toxicol. 2010 Mar;48(3):922-7. | | Butin reduces oxidative stress-induced mitochondrial dysfunction via scavenging of reactive oxygen species.[Pubmed: 20060874] | This study investigated the cytoprotective effect of Butin, a flavonoid, on hydrogen peroxide (H(2)O(2))-induced mitochondrial dysfunction.
METHODS AND RESULTS:
Electron spin resonance (ESR) spectrometry revealed Butin's significant scavenging effects on superoxide radicals and hydroxyl radicals. When H(2)O(2) was used to induce an increase in mitochondrial reactive oxygen species (ROS) in Chinese hamster lung fibroblast (V79-4) cells, Butin treatment decreased high level of ROS. Butin also attenuated intracellular Ca(2+) levels that have been induced by H(2)O(2). Furthermore, Butin recovered ATP levels and succinate dehydrogenase activity that had been decreased by H(2)O(2) treatment.
CONCLUSIONS:
We conclude these results suggest Butin decreased mitochondrial ROS accumulation, balanced intracellular Ca(2+) levels, and improved mitochondrial energy production, thus recovering mitochondrial function. | | Chem Biol Interact. 2009 Oct 30;181(3):338-42. | | Butin decreases oxidative stress-induced 8-hydroxy-2'-deoxyguanosine levels via activation of oxoguanine glycosylase 1.[Pubmed: 19631197] | In response to oxidative DNA base damage, oxoguanine glycosylase 1 (OGG1), in a base-excision repair (BER) pathway in mammals, plays a vital role in the repair of 8-hydroxy-2'-deoxyguanosine (8-OHdG), which is a reliable marker of reactive oxygen species (ROS)-induced DNA base modification and contributes to the pathologic process of cancer.Recently, we have shown that Butin (7,3',4'-trihydroxydihydroflavone) protects cells against hydrogen peroxide (H2O2)-induced damage of cellular components including DNA.
METHODS AND RESULTS:
In the present study, we examined the possible protective effect of Butin on oxidative stress-induced DNA base modification, especially 8-OHdG. Hydrogen peroxide significantly increased the level of 8-OHdG, which was detected by 8-OHdG ELISA and confocal microscopy, but Butin decreased this level. Suppression of 8-OHdG formation by Butin was related to the enhanced mRNA and protein expression of OGG1, which was detected by RT-PCR and Western blot analysis. Butin also increased the transcriptional activity of OGG1, which was suppressed by H2O2 treatment; this transcriptional activity was detected by OGG1 promoter luciferase assay.
Butin enhanced the expression of phosphorylated Akt (active form of Akt), a regulator of OGG1, which was decreased by H2O2 treatment.
CONCLUSIONS:
A PI3K-specific inhibitor, LY294002, abolished the phosphorylated Akt and OGG1 expressions induced by Butin, suggesting that OGG1 induction by Butin involves the PI3K/Akt pathway. |
|
| In vivo: |
| Sci Rep . 2017 Jan 27;7:41491. | | Protective effect of butin against ischemia/reperfusion-induced myocardial injury in diabetic mice: involvement of the AMPK/GSK-3β/Nrf2 signaling pathway[Pubmed: 28128361] | | Abstract
Hyperglycemia-induced reactive oxygen species (ROS) generation contributes to development of diabetic cardiomyopathy (DCM). This study was designed to determine the effect of an antioxidant Butin (BUT) on ischemia/reperfusion-induced myocardial injury in diabetic mice. Myocardial ischemia/reperfusion (MI/R) was induced in C57/BL6J diabetes mice. Infarct size and cardiac function were detected. For in vitro study, H9c2 cells were used. To clarify the mechanisms, proteases inhibitors or siRNA were used. Proteins levels were investigated by Western blotting. In diabetes MI/R model, BUT significantly alleviated myocardial infarction and improved heart function, together with prevented diabetes-induced cardiac oxidative damage. The expression of Nrf2, AMPK, AKT and GSK-3β were significantly increased by BUT. Furthermore, in cultured H9c2 cardiac cells silencing Nrf2 gene with its siRNA abolished the BUT's prevention of I/R-induced myocardial injury. Inhibition of AMPK and AKT signaling by relative inhibitor or specific siRNA decreased the level of BUT-induced Nrf2 expression, and diminished the protective effects of BUT. The interplay relationship between GSK-3β and Nrf2 was also verified with relative overexpression and inhibitors. Our findings indicated that BUT protected against I/R-induced ROS-mediated apoptosis by upregulating the AMPK/Akt/GSK-3β pathway, which further activated Nrf2-regulated antioxidant enzymes in diabetic cardiomyocytes exposed to I/R. | | Transl Neurosci . 2018 May 8;9:7-12. | | Butin Attenuates Brain Edema in a Rat Model of Intracerebral Hemorrhage by Anti Inflammatory Pathway[Pubmed: 29755784] | | Abstract
Background: This study evaluates the effect of Butin against brain edema in intracerebral hemorrhage (ICH).
Methodology: ICH was induced by injecting bacterial collagenase in the brain and all the animals were separated into four groups such as control group, ICH group treated with vehicle, Butin 25 and 50 mg/kg group receives Butin (25 and 50 mg/kg, i.p.)60 min after the induction of ICH in all animals. One day after neurological score, hemorrhagic injury and expressions of protein responsible for apoptosis and inflammatory cytokines were assessed in the brain tissue of ICH rats.
Result: Neurological scoring significantly increased and hemorrhagic lesion volume decreased in Butin treated group of rats compared to ICH group. However, treatment with Butin significantly decreases the ratio of Bax/Bcl-2 and protein expression of Cleaved caspase-3 than ICH group in dose dependent manner. Level of inflammatory mediators such as tumor necrosis factor-α (TNF-α) and interlukin-6 (IL-6) in the brain tissues were significantly decreased in the Butin treated group than ICH group. In addition Butin attenuates the altered signaling pathway of NF-κB in the brain tissues of ICH rats.
Conclusion: Our study concludes that Butin attenuates the altered behavior and neuronal condition in ICH rats by reducing apoptosis and inflammatory response.
Keywords: Brain edema; Butin; Cytokines; Inflammation; Intracerebral hemorrhage. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)