| In vitro: |
| Arch Pharm Res. 2016 May;39(5):713-20. | | Anti-osteoclastogenic effects of isoquinoline alkaloids from the rhizome extract of Sinomenium acutum.[Pubmed: 26992921] |
METHODS AND RESULTS:
A phytochemical investigation for the rhizome extract from Sinomenium acutum (Menispermaceae) resulted in the isolation of several active principles responsible for the anti-osteoclastogenic property of the extract, together with related isoquinoline alkaloids (1-13) including two new compounds, 1 and 2. Among isolated compounds, salutaridine (7), dauricumine (10), cheilanthifoline (12), and Dauriporphine (13) were observed to give significant inhibitions on receptor activator of nuclear factor-κB ligand-induced differentiation of mouse bone marrow-derived macrophages into multinucleated osteoclasts, respectively.
CONCLUSIONS:
The chemical structures of two newly isolated compounds, 1 and 2 were established as 8-demethoxycephatonine (1) and 7(R)-7,8-dihydrosinomenine (2), by spectroscopic analyses including 2D NMR experiments. | | Arch Pharm Res. 2006 Aug;29(8):627-32. | | Aporphine alkaloids and their reversal activity of multidrug resistance (MDR) from the stems and rhizomes of Sinomenium acutum.[Pubmed: 16964757] |
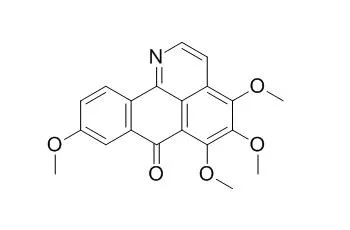
METHODS AND RESULTS:
Chromatographic separation of the MeOH extract from the stems and rhizomes of Sinomemium acutum led to the isolation of nine alkaloids and a lignan. Their structures were determined to be Dauriporphine (1), bianfugecine (2), dauriporphinoline (3), menisporphine (4), (-)-syringaresinol (5), N-feruloyltyramine (6), acutumine (7), dauricumine (8), sinomenine (9), and magnoflorine (10) by spectroscopic means. These compounds were examined for their P-gp mediated MDR reversal activity in human cancer cells.
CONCLUSIONS:
Compound 1 showed the most potent P-gp MDR inhibition activity with an ED50 value 0.03 microg/mL and 0.00010 microg/mL in the MES-SA/DX5 and HCT15 cells, respectively. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)