| In vitro: |
| Planta Med. 2013 Apr;79(6):499-505. | | Diarylheptanoids from Alnus glutinosa bark and their chemoprotective effect on human lymphocytes DNA.[Pubmed: 23512500] |
METHODS AND RESULTS:
A study of secondary metabolites from the bark of Alnus glutinosa led to the isolation of fourteen diarylheptanoids: oregonin (1), platyphylloside (2), rubranoside A (3), rubranoside B (4), Hirsutanonol (5), hirsutenone (6), Hirsutanonol-5-O-β-D-glucopyranoside (7), platyphyllonol-5-O-β-D-xylopyranoside (8), aceroside VII (9), alnuside A (10), alnuside B (11), 1,7-bis-(3,4-dihydoxyphenyl)-5-hydroxy-heptane-3-O-β-D-xylopyranoside (12), (5S)-1-(4-hydroxyphenyl)-7-(3,4-dihydroxyphenyl)-5-O-β-D-glucopyranosyl-heptan-3-one (13), and (5S)-1,7-bis-(3,4-dihydroxyphenyl)-5-O-β-D-[6-(3,4-dimethoxycinnamoylglucopyranosyl)]-heptan-3-one (14). All of the diarylheptanoids, except 1 and 5, were found in A. glutinosa for the first time, while 13 and 14 were new compounds. The structures were determined by spectroscopic techniques: 1D and 2D NMR, HR-ESI-MS, FTIR, UV, and CD. All isolated compounds were analyzed for an in vitro protective effect on chromosome aberrations in peripheral human lymphocytes using the cytokinesis-block micronucleus assay.
CONCLUSIONS:
The majority of them, including the new compounds 13 and 14, exerted a pronounced effect in decreasing DNA damage in human lymphocytes. Diarylheptanoids 1, 2, 5, 13, and 14 at a concentration of 1 µg/mL decreased the frequency of micronuclei by 52.8 %, 43.8 %, 63.6 %, 44.4 %, and 56.0 %, respectively, exerting a much stronger effect than the synthetic protector amifostine (17.2 %, c = 1 µg/mL). | | Arch Pharm Res. 2008 Oct;31(10):1287-9. | | Cytotoxic activities of diarylheptanoids from Alnus japonica.[Pubmed: 18958419] |
METHODS AND RESULTS:
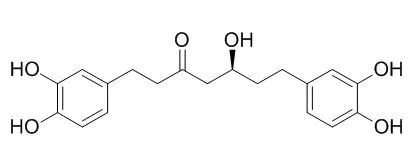
The diarylheptanoids (1-10) 1,7-bis-(3,4-dihydroxyphenyl)-heptane-3-O-beta-D-glucopyranosyl(1-->3)-beta-D-xylopyranoside (1), 1,7-bis-(3,4-dihydroxyphenyl)-heptane-3-O-beta-D-apiofuranosyl(1-->6)-beta-D-glucopyranoside (2), 1,7-bis-(3,4-dihydroxyphenyl)-heptane-5-O-beta-D-glucopyranoside (3), 1,7-bis-(3,4-dihydroxyphenyl)-5-hydroxyheptane (4), 1,7-bis-(3,4-dihydroxyphenyl)-heptane-3-one-5-O-beta-D-glucopyranoside (5), oregonin (6), Hirsutanonol (7), hirsutenone (8), 1,7-bis-(3,4-dihydroxyphenyl)-5-hydroxyheptane-3-O-beta-D-xylopyranoside (9), and platyphylloside (10), isolated from the bark of Alnus japonica, were analyzed for their cytotoxic activities on various human and mouse cancer cell lines. The cytotoxic activities of these ten compounds were evaluated against murine B16 melanoma, human SNU-1 gastric cancer, human SNU-354 hepatoma cancer and human SNU-C4 colorectal cell lines.
CONCLUSIONS:
The diarylheptanoids showed potent cytotoxic activities against murine B16 melanoma cells and human SNU-C1 gastric cancer cell when the cell viability was analyzed by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide) assay. | | Korean J. Medicinal Crop Sci.,2005,13(2):85-90. | | Constituents and their DPPH Scavenging Activities from the Leaves of Alnus hirsuta (Spach) Rupr.[Reference: WebLink] |
METHODS AND RESULTS:
Phytochemical study on the EtOAc fraction from a MeOH extract of the leaves of Alnus hirsuta Rupr. led to the isolation of nine compounds betulin (1), betulinic acid (2), Hirsutanonol (3), hirsutenone (4), quercetin (5), avicularin (6), gallic acid (7), hyperin (8), and daucosterol (9). Among them, six compounds 1, 2, 57, and 9 are report from this plant for the first time. All isolated compounds were evaluated for their antioxidant activity using DPPH radical scavenging capacity and inhibition effect on mitochondrial lipid peroxidation.
CONCLUSIONS:
Six phenolic compounds 3-8 were found to have potent antioxidant activity. Of which, compounds 3, 4 and 5 showed significant free radical scavenging activity with the values of , respectively. In addition, the compounds 3-8 exhibited inhibition effect on the mitochondrial lipid peroxidation with the values of , respectively. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)