| Description: |
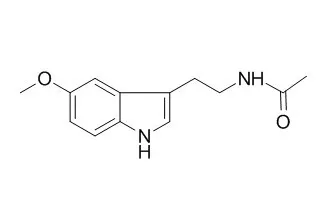
Melatonin, a hormone produced in the brain, is a potent melatonin receptor activator, and possesses important anti-cancer, antioxidative and anti-inflammatory properties,
it can reduce lead toxicity in vivo and in vitro. Melatonin may be useful as a pharmacological agent to protect against hepatic metabolic diseases due to its ability to regulate expression of miR-23a. |
| In vivo: |
| Biochem Biophys Res Commun. 2015 Mar 13;458(3):462-9. | | Melatonin ameliorates ER stress-mediated hepatic steatosis through miR-23a in the liver.[Pubmed: 25660457] | The endoplasmic reticulum (ER) stress induces hepatic steatosis and inflammation in the liver. Although Melatonin ameliorates ER stress-target genes, it remains unknown whether Melatonin protects against hepatic steatosis as well as inflammation through regulation of miRNA. MicroRNAs have been identified as pivotal regulators in the field of gene regulation and their dysfunctions are a common feature in a variety of metabolic diseases. Especially, among miRNAs, miR-23a has been shown to regulate ER stress.
METHODS AND RESULTS:
Herein, we investigated the crucial roles of Melatonin in hepatic steatosis and inflammation in vivo. Tunicamycin challenge caused increase of hepatic triglyceride and intracellular calcium levels through activation of ER stress, whereas these phenomena were partially disrupted by Melatonin. We also demonstrated that expression of miR-23a stimulated with tunicamycin was rescued by Melatonin treatment, resulting in reduced ER stress in primary hepatocytes.
CONCLUSIONS:
Overall, these results suggest a new function of Melatonin that is involved in ameliorating ER stress-induced hepatic steatosis and inflammation by attenuating miR-23a. Melatonin may be useful as a pharmacological agent to protect against hepatic metabolic diseases due to its ability to regulate expression of miR-23a. | | Toxicol Lett. 2015 Mar 4;233(2):78-83. | | Melatonin reduces lead levels in blood, brain and bone and increases lead excretion in rats subjected to subacute lead treatment.[Pubmed: 25601058] | Melatonin, a hormone known for its effects on free radical scavenging and antioxidant activity, can reduce lead toxicity in vivo and in vitro.We examined the effects of Melatonin on lead bio-distribution.
METHODS AND RESULTS:
Rats were intraperitoneally injected with lead acetate (10, 15 or 20mg/kg/day) with or without Melatonin (10mg/kg/day) daily for 10 days. In rats intoxicated with the highest lead doses, those treated with Melatonin had lower lead levels in blood and higher levels in urine and feces than those treated with lead alone, suggesting that Melatonin increases lead excretion. To explore the mechanism underlying this effect, we first assessed whether lead/Melatonin complexes were formed directly. Electronic density functional (DFT) calculations showed that a lead/Melatonin complex is energetically feasible; however, UV spectroscopy and NMR analysis showed no evidence of such complexes. Next, we examined the liver mRNA levels of metallothioneins (MT) 1 and 2. Melatonin cotreatment increased the MT2 mRNA expression in the liver of rats that received the highest doses of lead. The potential effects of MTs on the tissue distribution and excretion of lead are not well understood.
CONCLUSIONS:
This is the first report to suggest that Melatonin directly affects lead levels in organisms exposed to subacute lead intoxication. | | Anticancer Res. 2014 Dec;34(12):7327-37. | | Melatonin in patients with cancer receiving chemotherapy: a randomized, double-blind, placebo-controlled trial.[Pubmed: 25503168] | The MIRCIT trial was a randomized, double-blind, placebo-controlled study of advanced Non-small cell lung cancer (NSCLC).
METHODS AND RESULTS:
Patients were randomized to receive 10 mg or 20 mg of Melatonin or placebo. Assessment of health-related quality of life (HRQoL) was completed at baseline, and at 2, 3 and 7 months. Survival and adverse events were collected. DNA damage marker 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG) was measured during the first three months of chemotherapy.
Patients in the Melatonin-treated group had better adjusted HRQoL scores, with a slightly significantly better score (2.69 points, 95% confidence interval (CI)=0.01-5.38, p=0.049) being found in social well-being. Median survival was 7.3 months (95% CI=3.42-11.14) without significant difference. A great amont of DNA damage marker was observed in the placebo-treated group, and this was associated with lower survival (r(2)=-0.656, p=0.02), implying the protective effect of Melatonin in healthy cells.
CONCLUSIONS:
Melatonin in combination with chemotherapy did not affect survival and adverse events of advanced patients with NSCLC, but there was a trend for better HRQoL. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)