| Structure Identification: |
| Acta Crystallogr Sect E Struct Rep Online. 2011 Jul 1;67(Pt 7):o1706-7. | | Absolute configuration of micromelin.[Pubmed: 21837101] |
METHODS AND RESULTS:
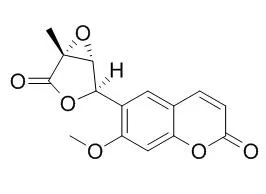
The title compound Micromelin{systematic name: 7-meth-oxy-6-[(1R,2R,5R)-5-methyl-4-oxo-3,6-dioxabicyclo-[3.1.0]hexan-2-yl]-2H-chromen-2-one}, C(15)H(12)O(6), is a coumarin, which was isolated from the roots of Micromelum glanduliferum. There are two mol-ecules in the asymmetric unit with slight differences in bond angles. In both mol-ecules, the furan ring adopts a flattened envelope conformation. In the crystal, mol-ecules are linked by weak C-H⋯O inter-actions into chains along the a axis. Aromatic π-π stacking inter-actions with centroid-centroid distances in the range 3.6995 (11)-3.8069 (11) Å and C⋯O short contacts [3.030 (2)-3.171 (3) Å] also occur. | | Arch Pharm Res. 2011 Apr;34(4):527-31. | | C-7 oxygenated coumarins from the fruits of Micromelum minutum.[Pubmed: 21544717] |
METHODS AND RESULTS:
A new 7-oxygenated coumarin, 7-demethylmurralonginol isovalerate (1), and a new natural product, murralonginol (2), together with seven known 7-oxygenated coumarins, murralonginol isovalerate (3), murralongin (4), Micromelin (5), scopoletin (6), microminutin (7), murrangatin (8), and minumicrolin (9), were isolated from the fruits of Micromelum minutum. The structures of these compounds were established on the basis of their 1D and 2D NMR spectroscopic data.
CONCLUSIONS:
Among these isolates, compounds 2 and 4 - 9 exhibited cytotoxicity against cholangiocarcinoma cell line, KKU-100. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)