| In vivo: |
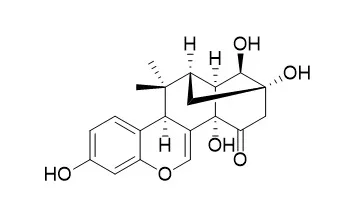
| Phytother Res . 2018 Feb;32(2):365-369. | | A pilot pharmacokinetic study of miroestrol and deoxymiroestrol on rabbit sera using polyclonal antibody-based icELISA analysis[Pubmed: 29168310] | | Miroestrol (ME) and deoxyMiroestrol (DME) are the most potent phytoestrogens and bioactive markers in Pueraria candollei var. mirifica tuberous roots. To understand their pharmacokinetic profiles, a pharmacokinetic study of ME and DME, at 0.43 and 0.21 mg per kg body weight, respectively, in three rabbits was performed after orally administering a single dose of P. candollei var. mirifica enriched fraction extract. Two established polyclonal antibody-based indirect competitive enzyme-linked immunosorbent assays were validated to determine ME and DME in rabbit sera. In rabbits, the area under the 0- to 48-hr concentration-time curve of ME and DME were 854.92 and 1,692.84 ng·h/ml, respectively. The maximum concentration of ME was measured 1 hr after administration as 69.62 ± 8.28 ng/ml, and the maximum concentration of DME was measured at 3 hr as 81.8 ± 5.43 ng/ml. These results provide an initial approach for designing and studying the relationship between the ME and DME levels and their therapeutic effects based on their pharmacokinetic profiles. | | Fitoterapia . 2012 Dec;83(8):1687-92. | | Impact of Pueraria candollei var. mirifica and its potent phytoestrogen miroestrol on expression of bone-specific genes in ovariectomized mice[Pubmed: 23041523] | | Miroestrol (MR) is a highly active phytoestrogen isolated from tuberous root of Pueraria candollei var. mirifica (PM). Modulatory effects of PM and MR on osteoprotegerin (OPG) and receptor activator of nuclear factor kappa B ligand (RANKL) mRNAs which are bone-specific genes were investigated in ovariectomized female ICR mice. After ovariectomy, expression of OPG mRNA was suppressed but that of RANKL was induced. Estradiol benzoate (E2) recovered OPG expression to the level comparable to the sham while that of RANKL was suppressed in ovariectomized mice. PM crude extract (PME) significantly down-regulated the expression of RANKL mRNA with no change in the OPG level whereas MR elevated the expression of OPG mRNA with lowering level of RANKL mRNA, resulting in the increased OPG/RANKL ratio, and consequently lead to lowering progression of osteoporosis at molecular level. These findings revealed potential of PME and MR on bone loss prevention via increasing the ratio of OPG to RANKL (osteoformation/osteoresorption) in liver of ovariectomized mice. Therefore, using PME and MR as alternative hormone replacement therapy of E2 might be beneficial recommended due to advantageous on regulation of osteoporosis related genes. | | Phytomedicine . 2014 Sep 25;21(11):1249-1255. | | Effect of miroestrol on ovariectomy-induced cognitive impairment and lipid peroxidation in mouse brain[Pubmed: 25172786] | | Miroestrol (MR) is a phytoestrogen isolated from Pueraria candollei var. mirifica (KwaoKrueaKhao), a Thai medicinal plant used for rejuvenation. We examined the effects of MR on cognitive function, oxidative brain damage, and the expression of genes encoding brain-derived neurotrophic factor (BDNF) and cyclic AMP-responsive element-binding protein (CREB), factors implicated in neurogenesis and synaptic plasticity, in ovariectomized (OVX) mice. OVX decreased serum 17β-estradiol level and uterine weight. OVX also impaired object recognition performance in the novel object recognition test and spatial cognitive performance in the Y-maze test and the water maze test. Daily treatment of MR dose-dependently attenuated OVX-induced cognitive dysfunction. Moreover, OVX mice had a significantly increased level of thiobarbituric acid-reactive substances, and down-regulated expression levels of BDNF and CREB mRNAs in the hippocampus and frontal cortex. MR treatment as well as hormone replacement therapy with 17β-estradiol significantly reversed these neurochemical alterations caused by OVX. These results suggest that MR ameliorates cognitive deficits in OVX animals via attenuation of OVX-induced oxidative stress and down-regulation of BDNF and CREB mRNA transcription in the brain. Our findings raise the possibility that MR and Pueraria candollei var. mirifica, the plant of origin of MR, may have a beneficial effect on cognitive deficits like AD in which menopause/ovariectomy are implicated as risk factors. | | J Pharm Pharmacol . 2016 Apr;68(4):475-484. | | Regulation of cancer-related genes - Cyp1a1, Cyp1b1, Cyp19, Nqo1 and Comt - expression in β-naphthoflavone-treated mice by miroestrol[Pubmed: 26893163] | | Objective: The effects of Miroestrol (MR), an active phytoestrogen from Pueraria candollei var. mirifica, on expression of cancer-related genes were determined.
Methods: Seven-week-old female ICR mice (n = 5 each) were subcutaneously administered estradiol (E2, 0.5 mg/kg/day) or MR (0.5 or 5 mg/kg/day) daily for 7 days. Some were given ER or MR in combination with β-naphthoflavone (BNF, 30 mg/kg/day) for the last 3 days. The expression of cancer-related genes including cytochrome P450 1A (Cyp1a), cytochrome P450 1B1 (Cyp1b1), aromatase P450 (Cyp19), NAD(P)H: quinone oxidoreductase 1 (Nqo1) and catechol-O-methyltransferase (Comt) were evaluated.
Key findings: In the presence of BNF, MR suppressed hepatic CYP1A1 activity and CYP1A2 activity, expression of CYP1B1 mRNA and expression of CYP1A1/2 and CYP1B1 protein. E2, by contrast, did not. MR restored expression levels of hepatic NQO1 and uterine COMT in BNF-treated mice. Furthermore, MR increased expression of uterine CYP19 to the same extent as E2.
Conclusion: MR may be superior to E2 as it downregulates expression of CYP1. Moreover, MR normalized expression of both NQO1 and COMT, the protective enzymes, in murine liver and uteri. These results support the use of MR as an alternative supplement for menopausal women, MR having the extra benefit of reducing cancer risk. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)