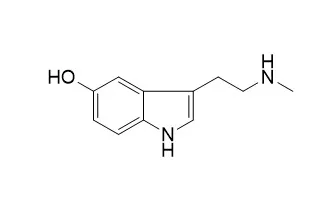
| Description: |
N-methylserotonin, orally administered to germ-free mice, reduced adiposity, altered liver glycogenesis, shortened gut transit time, and changed expression of genes that regulate circadian rhythm in the liver and colon. N-Methylserotonin exerted antiendotoxin inflammatory effect via suppressing the TLR4/MyD88/NF-κB and TLR4/MyD88/MAPKs signaling pathway. Meanwhile,N-Methylserotonin could also interfere the metabolism of SM, Cer and PC probably by regulating LBP, PLA2, CERK, SMPD and SGMS.
|
| In vitro: |
| Cell . 2022 Jul 7;185(14):2495-2509.e11. | | Microbial liberation of N-methylserotonin from orange fiber in gnotobiotic mice and humans[Pubmed: 35764090] | | Plant fibers in byproduct streams produced by non-harsh food processing methods represent biorepositories of diverse, naturally occurring, and physiologically active biomolecules. To demonstrate one approach for their characterization, mass spectrometry of intestinal contents from gnotobiotic mice, plus in vitro studies, revealed liberation of N-Methylserotonin from orange fibers by human gut microbiota members including Bacteroides ovatus. Functional genomic analyses of B. ovatus strains grown under permissive and non-permissive N-Methylserotonin "mining" conditions revealed polysaccharide utilization loci that target pectins whose expression correlate with strain-specific liberation of this compound. N-Methylserotonin, orally administered to germ-free mice, reduced adiposity, altered liver glycogenesis, shortened gut transit time, and changed expression of genes that regulate circadian rhythm in the liver and colon. In human studies, dose-dependent, orange-fiber-specific fecal accumulation of N-Methylserotonin positively correlated with levels of microbiome genes encoding enzymes that digest pectic glycans. Identifying this type of microbial mining activity has potential therapeutic implications. | | Neuroreport . 1995 Nov 27;6(17):2378-2380. | | Bufotenine reconsidered as a diagnostic indicator of psychiatric disorders[Pubmed: 8747157] | | We have analyzed products of the serotonin-degradative pathway, in which both N-Methylserotonin and bufotenine are formed in urine specimens of products with psychiatric disorders by three-dimensional HPLC with electrochemical detection. Bufotenine was detected in urine from all autistic patients with mental retardation and epilepsy (n = 18) and many autistic patients (32/47) with mental retardation. Bufotenine was detected in the urine of 15 of 18 patients with depression. Thirteen of 15 schizophrenic patients were also positive for bufotenine. N-Methylserotonin was also detected in some cases of each disorder. Only two of 200 urine specimens from healthy controls were positive for bufotenine. Thus, the presence and levels of bufotenine might be useful and important markers of some psychiatric disorders. | | Zhongguo Zhong Yao Za Zhi . 2021 Sep;46(18):4774-4781. | | [Study on anti-inflammatory activity and mechanism of indolealkylamines in toad skin on LPS-activated neutrophils][Pubmed: 34581088] | | Indolealkylamines(IAAs) are the main hydrophilic substances in toad skin, mainly including free N-methyl-5-hydroxytryptamine, bufotenine, bufotenidine, dehydrobufotenine, and binding bufothionine. In this study, the LPS-activated neutrophils were used to investigate the structure-activity relationship and anti-inflammatory mechanism of the above-mentioned five monomers from the toad skin in vitro. The neutrophils were divided into the control group, model group(1 μg·mL~(-1) LPS), positive drug group(100 μg·mL~(-1) indometacin), as well as the low-(50 μg·mL~(-1)), medium-(100 μg·mL~(-1)) and high-dose(200 μg·mL~(-1)) free N-methyl-5-hydroxytryptamine, bufotenine, bufotenidine, dehydrobufotenine, and binding bufothionine groups. The levels of IL-6, TNF-α and IL-1β in the neutrophil supernatant of each group was measured by enzyme-linked immunosorbent assay(ELISA) after LPS stimulation, followed by the detection of apoptosis in each group after Annexin V/PI staining. The protein expression levels of caspase-3, Bax, Bcl-2, beclin1, LC3-I, and LC3-Ⅱ were assayed by Western blot. The results showed that IAAs reduced the excessive secretion of inflammatory cytokines caused by LPS compared with the model group. Besides, the activity of each free IAAs(N-methyl-5-hydroxytryptamine, bufotenine, bufotenidine and dehydrobufotenine), especially bufotenine, was stronger than that of the binding bufothionine. As revealed by Annexin V/PI staining, LPS delayed the early apoptosis of neutrophils compared with the control group, while bufotenine promoted the apoptosis of neutrophils in a dose-dependent manner, which might be related to the elevated expression of apoptosis-related protein Bax/Bcl-2. In addition, LPS activated the autophagy pathways in neutrophils. This study confirmed the efficacy of IAAs in reducing the secretion of inflammatory cytokines in neutrophils induced by LPS for the first time. For instance, bufotenine exerts the anti-inflammatory effect possibly by inducing the apoptosis of neutrophils. | | J Ethnopharmacol . 2021 Apr 6;269:113677. | | Evaluation of analgesic and anti-inflammatory actions of indolealkylamines from toad venom in mice using lipidomics and molecular docking[Pubmed: 33321188] | | Ethnopharmacological relevance: Toad venom is one of widely used traditional Chinese medicines due to its analgesic and anti-inflammatory activities. However, hydrophilic alkaloids from toad venom, which may have certain pharmacological activities, have not been systematic studied.
Aim of the study: The aim of the study was to identify the indolealkylamines (IAAs) from toad venom and investigate the analgesic and anti-inflammatory actions.
Materials and methods: The alkaloids were extracted and identified by high-resolution mass spectrometry. The analgesic abilities were determined using hot-plate test, formalin test and von Frey test. High-sensitivity lipidomics was used to investigate the regulatory function of IAAs on inflammatory eicosanoids. Besides, network pharmacology and molecular docking were used to demonstrate the candidate targets of IAAs.
Results: 22 constituents have been characterized by high performance liquid chromatography (HPLC)-Triple TOF 5600, including six specific IAAs (serotonin, N-methyl serotonin, bufotenine, bufotenidine, bufothionine and dehydrobufotenine). Pharmacological studies showed that the IAAs from toad venom exerted significant analgesic activities at doses of 5, 15 and 45 mg/kg in vivo. Moreover, lipids analysis revealed IAAs might down-regulate inflammatory mediators from COX, LOX, DHA and LA pathways in formalin models, thus showing anti-inflammatory effect. The potent pharmacological function might because of the binding of IAAs and protein targets, such as sigma-1 receptor.
Conclusion: The studies provided a systemic evidence for the analgesic and anti-inflammatory activities of IAAs from toad venom. It suggested that IAAs might be a potential candidate to reduce inflammatory pain disorders. |
|
| In vivo: |
| Cell . 2022 Jul 7;185(14):2495-2509.e11. | | Microbial liberation of N-methylserotonin from orange fiber in gnotobiotic mice and humans[Pubmed: 35764090] | | Plant fibers in byproduct streams produced by non-harsh food processing methods represent biorepositories of diverse, naturally occurring, and physiologically active biomolecules. To demonstrate one approach for their characterization, mass spectrometry of intestinal contents from gnotobiotic mice, plus in vitro studies, revealed liberation of N-Methylserotonin from orange fibers by human gut microbiota members including Bacteroides ovatus. Functional genomic analyses of B. ovatus strains grown under permissive and non-permissive N-Methylserotonin "mining" conditions revealed polysaccharide utilization loci that target pectins whose expression correlate with strain-specific liberation of this compound. N-Methylserotonin, orally administered to germ-free mice, reduced adiposity, altered liver glycogenesis, shortened gut transit time, and changed expression of genes that regulate circadian rhythm in the liver and colon. In human studies, dose-dependent, orange-fiber-specific fecal accumulation of N-Methylserotonin positively correlated with levels of microbiome genes encoding enzymes that digest pectic glycans. Identifying this type of microbial mining activity has potential therapeutic implications. | | Psychopharmacology (Berl) . 2009 Oct;206(3):479-489. | | Alterations in tryptophan and purine metabolism in cocaine addiction: a metabolomic study[Pubmed: 19649617] | | Background: Mapping metabolic "signatures" can provide new insights into addictive mechanisms and potentially identify biomarkers and therapeutic targets.
Objective: We examined the differences in metabolites related to the tyrosine, tryptophan, purine, and oxidative stress pathways between cocaine-dependent subjects and healthy controls. Several of these metabolites serve as biological indices underlying the mechanisms of reinforcement, toxicity, and oxidative stress.
Methods: Metabolomic analysis was performed in 18 DSM-IV-diagnosed cocaine-dependent individuals with at least 2 weeks of abstinence and ten drug-free controls. Plasma concentrations of 37 known metabolites were analyzed and compared using a liquid chromatography electrochemical array platform. Multivariate analyses were used to study the relationship between severity of drug use [Addiction Severity Index (ASI) scores] and biological measures.
Results: Cocaine subjects showed significantly higher levels of N-Methylserotonin (p < 0.0017) and guanine (p < 0.0031) and lower concentrations of hypoxanthine (p < 0.0002), anthranilate (p < 0.0024), and xanthine (p < 0.012), compared to controls. Multivariate analyses showed that a combination of N-Methylserotonin and xanthine contributed to 73% of the variance in predicting the ASI scores (p < 0.0001). Logistic regression showed that a model combining N-Methylserotonin, xanthine, xanthosine, and guanine differentiated cocaine and control groups with no overlap.
Conclusions: Alterations in the methylation processes in the serotonin pathways and purine metabolism seem to be associated with chronic exposure to cocaine. Given the preliminary nature and cross-sectional design of the study, the findings need to be confirmed in larger samples of cocaine-dependent subjects, preferably in a longitudinal design. | | J Ethnopharmacol . 2019 Oct 28;243:112122. | | The protection of indolealkylamines from LPS-induced inflammation in zebrafish[Pubmed: 31356965] | | Ethnopharmacological relevance: Toad skin came from Bufo bufo gargarizans Cantor and Bufo melanostictus Schneider. As the traditional Chinese medicine, it had the effect of clearing away heat and detoxification. In traditional applications, toad skin was often used for the treatment of cancer and inflammation. Total indolealkylamines (IAAs) from this medicine were proved the main compounds exert anti-inflammatory activity in our previous research.
Aim of the study: In the present study, we aimed to investigate the potential mechanism of anti-inflammatory activity of IAAs on LPS induced zebrafish.
Materials and methods: LPS induced zebrafish was applicated as an in vivo inflammation model to clarify the structure-activity relationship of 4 major IAAs (N-methyl serotonin, bufotenine, dehydrobufotenine and bufothionine) from toad skin. Quantitative RT-PCR was applied to detect key cytokines and members of the MyD88-dependent signaling pathway. In addition, the targeted lipidomics was conducted to find out the potential biomarkers in the inflammatory zebrafish. Network pharmacology was used to unveil the main enzymes closely related to the target lipids.
Results: Our results showed that the anti-inflammatory activity of free IAAs (N-methyl serotonin, bufotenine and dehydrobufotenine) was more potent than that of combined IAAs (bufothionine). RT-PCR demonstrated that 4 IAAs exerted antiendotoxin inflammatory effect via suppressing the TLR4/MyD88/NF-κB and TLR4/MyD88/MAPKs signaling pathway. A total of 33 possible inflammatory biomarkers, including 14 SM, 6 Cer, 11 PC and 2 GlcCer, triggered by LPS were screened out. The levels of most of candidates could be regulated toward a normal level by IAAs, especially in N-methyl serotonin and dehydrobufotenine groups. Enzymes especially LBP, PLA2, CERK, SMPD and SGMS were found closely associated with the regulation of most lipid markers.
Conclusions: Overall, the mechanism underlying the anti-inflammatory activity of IAAs probably attributed to their capability to suppress NF-κB and MAPKs inflammatory pathway. Meanwhile, IAAs could also interfere the metabolism of SM, Cer and PC probably by regulating LBP, PLA2, CERK, SMPD and SGMS. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)