| Structure Identification: |
| Agricultural & Biological Chemistry,1976, 40(6):1113-8. | | The structure of piperenone[Reference: WebLink] |
METHODS AND RESULTS:
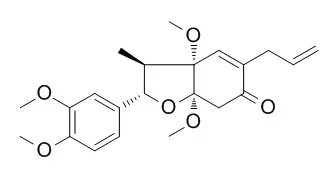
The structure of a new neolignan, Piperenone, isolated as an insect antifeeding substance from Piper futokadzura Sieb. et Zucc. has been determined on the basis of chemical and spectral evidence. It is shown to be 5-allyl-3a, 7a-dimethoxy-2-(3, 4-dimethoxyphenyl)-3-methyl-2, 3, 3a, 6, 7, 7a-hexahydro-6-oxobenzofuran (I). Both the absolute configurations at C-2 and C-3, two of four asymmetric carbons in I are deduced to be S-arrangements. | | Acta Chemica Scandinavica,1995,49 (2):142-8. | | Neolignans from Piper schmidtii and reassignment of the structure of schmiditin.[Reference: WebLink] |
METHODS AND RESULTS:
A new neolignan, (7R,8S,1'S)-A8'-1',4'-dihydro-3,4,5'-trimethoxy-4'-oxo-8.1',7.0.2' -lignan [(2R,3S,3aS)-2-(3,4-dimethoxyphenyl)-3,3a-dihydro-5-methoxy-3-methyl-3a-(2-propenyl)-2-benzofuran-6(2H)-one] (1), together with five known neolignans and a known alkaloid were isolated from the stems of Piper schmidtii Hook f. The known compounds were identified as kadsurin B (3), Piperenone (4), (7R,8S, 1'S)-A 8' -1',4'-dihydro-5' -methoxy- 3,4-methylenedioxy-4'oxo-8.1',7.0.2'-lignan (5), lancifolin C (6), lancifolin D (7) and an alkaloid, 1-cinnamoylpyrrolidine (2). The earlier proposed structure of schmiditin (12) was revised to (7S,8R,3'S,4'R,6'S)-88'-3',4',5',6'-tetrahydro-6' -hydroxy-3',4'dimethoxy-3,4- methylenedioxy-8.3',7.0.4' -lignan [(2S,3R,3aS,6S,7aR)-2-(l,3-benzodioxol-5-yl)- 2,3,3a,6,7,7a-hexahydro-3a,7a-dimethoxy-3-methyl-5-(2-propenyl)-2-benzofuran-6-ol= kadsurin B] (3) on the basis of spectral data and chemical transformations.
CONCLUSIONS:
Reassignment of the 1H NMR data of lancifolin C (6), the absolute stereochemistry of kadsurin A (10) and 13C NMR data of Piperenone (4) and lancifolin C (6) are also reported. In addition, methyl piperate (8) was isolated from the fruits. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)