| In vitro: |
| J. Org. Chem., 1999, 64 (7):2331–9. | | Rubrosides A−H, New Bioactive Tetramic Acid Glycosides from the Marine Sponge Siliquariaspongia japonica1[Reference: WebLink] |
METHODS AND RESULTS:
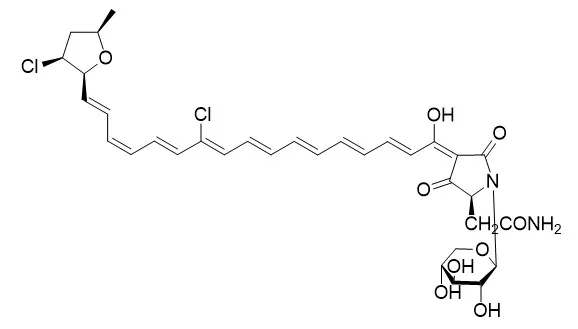
Eight new tetramic acid glycosides named rubrosides A-H(rubroside A,rubroside B, rubroside C, rubroside D, rubroside E, rubroside F, Rubroside G, rubroside H) have been isolated from the marine sponge Siliquariaspongia japonica. Their structures were elucidated on the basis of spectral data as tetramic acid glycosides containing polyenes terminating in a 4-chloro-2-methyltetrahydrofuran ring. The absolute stereochemistry of the furan functionality in the two major metabolites, rubrosides D and F, was determined by the NMR method using chiral anisotropic reagents for tetrahydro-2-furoic acid derived by RuO4 oxidation. The absolute stereochemistry of tetramic acid and of the sugar moieties in all rubrosides was deduced by chiral GC analysis of chemical degradation products. The rubrosides induced numerous large intracellular vacuoles in 3Y1 rat fibroblasts at concentrations of 0.5−1.0 μg/mL, and rubrosides A, C, D, and E were cytotoxic against P388 murine leukemia cells with IC50 values of 0.046−0.21 μg/mL.
CONCLUSIONS:
Most rubrosides show antifungal activity against Aspergillus fumigatus and Candida albicans. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)