| Kinase Assay: |
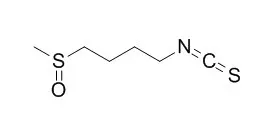
| Cancer Lett. 2014 Aug 28;351(1):41-9. | | Sulforaphane inhibits phorbol ester-stimulated IKK-NF-κB signaling and COX-2 expression in human mammary epithelial cells by targeting NF-κB activating kinase and ERK.[Pubmed: 24747121] | Sulforaphane, an isothiocyanate present in cruciferous vegetables, has been reported to possess anti-inflammatory and cancer chemopreventive properties. However, the molecular mechanisms by which Sulforaphane suppresses inflammation and carcinogenesis are yet to be fully elucidated. Since the aberrant expression of cyclooxygenase-2 (COX-2) links inflammation and cancer, the present study was aimed to elucidate the mechanisms by which Sulforaphane modulates COX-2 overexpression in human mammary epithelial (MCF-10A) cells stimulated with a prototypic tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA).
METHODS AND RESULTS:
Treatment of MCF-10A cells with Sulforaphane significantly inhibited TPA-induced expression of COX-2 protein and its mRNA transcript. Transient transfection of cells with deletion mutant constructs of COX-2 promoter revealed that the transcription factor nuclear factor-kappaB (NF-κB) plays a key role in TPA-induced COX-2 expression in MCF-10A cells. Pretreatment with Sulforaphane significantly attenuated nuclear localization, DNA binding and the transcriptional activity of NF-κB through inhibition of phosphorylation and subsequent degradation of IκBα in MCF-10A cells stimulated with TPA. Sulforaphane also attenuated TPA-induced activation of IκB kinases (IKK), NF-κB-activating kinase (NAK) and extracellular signal-regulated kinase-1/2 (ERK1/2). Pharmacological inhibition of IKK or transient transfection of cells with dominant-negative mutant forms of this kinase abrogated TPA-induced NF-κB activation and COX-2 expression. In addition, the blockade of ERK1/2 activation negated the catalytic activity of IKKα, but not that of IKKβ, whereas silencing NAK by specific siRNA abrogated the IKKβ activity in TPA-treated cells.
CONCLUSIONS:
Taken together, Sulforaphane inhibits TPA-induced NF-κB activation and COX-2 expression in MCF-10A cells by blocking two distinct signaling pathways mediated by ERK1/2-IKKα and NAK-IKKβ. | | Int J Oncol. 2014 Oct;45(4):1497-506. | | Sulforaphane induces apoptosis in T24 human urinary bladder cancer cells through a reactive oxygen species-mediated mitochondrial pathway: the involvement of endoplasmic reticulum stress and the Nrf2 signaling pathway.[Pubmed: 24993616] | Sulforaphane, a naturally occurring isothiocyanate found in cruciferous vegetables, has received a great deal of attention because of its ability to inhibit cell proliferation and induce apoptosis in cancer cells. In this study, we investigated the anticancer activity of Sulforaphane in the T24 human bladder cancer line, and explored its molecular mechanism of action.
METHODS AND RESULTS:
Our results showed that treatment with Sulforaphane inhibited cell viability and induced apoptosis in T24 cells in a concentration-dependent manner. Sulforaphane-induced apoptosis was associated with mitochondria dysfunction, cytochrome c release and Bcl-2/Bax dysregulation. Furthermore, the increased activity of caspase-9 and -3, but not caspase-8, was accompanied by the cleavage of poly ADP-ribose polymerase, indicating the involvement of the mitochondria-mediated intrinsic apoptotic pathway. Concomitant with these changes, Sulforaphane triggered reactive oxygen species (ROS) generation, which, along with the blockage of Sulforaphane-induced loss of mitochondrial membrane potential and apoptosis, was strongly attenuated by the ROS scavenger N-acetyl-L-cysteine. Furthermore, Sulforaphane was observed to activate endoplasmic reticulum (ER) stress and the nuclear factor-E2-related factor-2 (Nrf2) signaling pathway, as demonstrated by the upregulation of ER stress‑related proteins, including glucose-regulated protein 78 and C/EBP-homologous protein, and the accumulation of phosphorylated Nrf2 proteins in the nucleus and induction of heme oxygenase-1 expression, respectively.
CONCLUSIONS:
Taken together, these results demonstrate that Sulforaphane has antitumor effects against bladder cancer cells through an ROS-mediated intrinsic apoptotic pathway, and suggest that ER stress and Nrf2 may represent strategic targets for Sulforaphane-induced apoptosis. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)